
$Pb{{(N{{O}_{3}})}_{2}}$ on heating gives a brown gas which undergoes dimerization on cooling? Identify the gas.
Answer
575.7k+ views
Hint: When lead nitrate is heated a brown coloured gas is formed which is the main constituent of photochemical smog. It can be used to prepare nitric acid and is also used in fertilizers. A dioxide group is present.
Complete Answer:
In order to answer our question, let us study a few things about lead nitrate. Lead nitrate has the molecular formula $Pb{{(N{{O}_{3}})}_{2}}$. It is soluble in water and occurs as a white powder, or a colourless crystal. Lead nitrate was used as starting materials and ingredients for lead paints, in Europe. It was also used in the nylon and polyester industry, and in the extraction of gold using the cyanide process. However, it is very toxic to humans and can affect the lungs and skin adversely. In order to manufacture lead nitrate, lead oxide can be reacted with concentrated nitric acid, or by reacting lead with dilute nitric acid:
\[\begin{align}
& PbO+2HN{{O}_{3}}(conc)\to Pb{{(N{{O}_{3}})}_{2}}\downarrow +{{H}_{2}}O \\
& Pb+4HN{{O}_{3}}(dil)\to Pb{{(N{{O}_{3}})}_{2}}+2N{{O}_{2}}+2{{H}_{2}}O \\
\end{align}\]
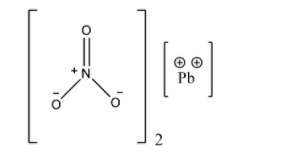
Lead nitrate has a face-centered cubic structure which has a cube length of about 784 picometres. In this configuration, every lead atom is bonded with 12 oxygen atoms, and all bond lengths of nitrogen-oxygen are identical. There is no possibility of rotation of nitrate groups at high temperatures, within the crystal lattice. Here is how lead nitrate looks like:
Now, let us come to our question. Lead nitrate decomposes to give a brown coloured gas which is nitrogen dioxide. Nitrogen dioxide has the formula $N{{O}_{2}}$. It is used in fertilisers and also occurs during the photochemical smog, where surroundings appear brownish. Nitric acid can be prepared from nitrogen dioxide. It is soluble in water. However, nitrogen dioxide can dimerize too. On dimerization, it produces ${{N}_{2}}{{O}_{4}}$. The reactions are:
\[\begin{align}
& 2Pb{{(N{{O}_{3}})}_{2}}\to 2PbO+4N{{O}_{2}}+{{O}_{2}} \\
& 2N{{O}_{2}}\to {{N}_{2}}{{O}_{4}} \\
\end{align}\]
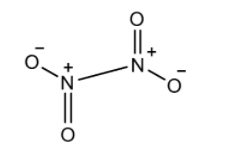
Note: The compound ${{N}_{2}}{{O}_{4}}$ is known as nitrogen tetroxide. Nitrogen dioxide is a brown coloured gas, but this is a red coloured liquid and has an unpleasant odour. The structure of ${{N}_{2}}{{O}_{4}}$ is:
Complete Answer:
In order to answer our question, let us study a few things about lead nitrate. Lead nitrate has the molecular formula $Pb{{(N{{O}_{3}})}_{2}}$. It is soluble in water and occurs as a white powder, or a colourless crystal. Lead nitrate was used as starting materials and ingredients for lead paints, in Europe. It was also used in the nylon and polyester industry, and in the extraction of gold using the cyanide process. However, it is very toxic to humans and can affect the lungs and skin adversely. In order to manufacture lead nitrate, lead oxide can be reacted with concentrated nitric acid, or by reacting lead with dilute nitric acid:
\[\begin{align}
& PbO+2HN{{O}_{3}}(conc)\to Pb{{(N{{O}_{3}})}_{2}}\downarrow +{{H}_{2}}O \\
& Pb+4HN{{O}_{3}}(dil)\to Pb{{(N{{O}_{3}})}_{2}}+2N{{O}_{2}}+2{{H}_{2}}O \\
\end{align}\]
Lead nitrate has a face-centered cubic structure which has a cube length of about 784 picometres. In this configuration, every lead atom is bonded with 12 oxygen atoms, and all bond lengths of nitrogen-oxygen are identical. There is no possibility of rotation of nitrate groups at high temperatures, within the crystal lattice. Here is how lead nitrate looks like:

Now, let us come to our question. Lead nitrate decomposes to give a brown coloured gas which is nitrogen dioxide. Nitrogen dioxide has the formula $N{{O}_{2}}$. It is used in fertilisers and also occurs during the photochemical smog, where surroundings appear brownish. Nitric acid can be prepared from nitrogen dioxide. It is soluble in water. However, nitrogen dioxide can dimerize too. On dimerization, it produces ${{N}_{2}}{{O}_{4}}$. The reactions are:
\[\begin{align}
& 2Pb{{(N{{O}_{3}})}_{2}}\to 2PbO+4N{{O}_{2}}+{{O}_{2}} \\
& 2N{{O}_{2}}\to {{N}_{2}}{{O}_{4}} \\
\end{align}\]
Note: The compound ${{N}_{2}}{{O}_{4}}$ is known as nitrogen tetroxide. Nitrogen dioxide is a brown coloured gas, but this is a red coloured liquid and has an unpleasant odour. The structure of ${{N}_{2}}{{O}_{4}}$ is:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




