
\[{{\text{P}}_{4}}{{\text{S}}_{3}}\] has 6 P-S and ____ P-P bonds.
Answer
569.7k+ views
Hint: Phosphorus sesquisulfide, \[{{\text{P}}_{4}}{{\text{S}}_{3}}\], an inorganic compound, with an orthorhombic crystal structure. By studying its geometry, we can determine the number of different types of bonds it has.
Complete Solution :
\[{{\text{P}}_{4}}{{\text{S}}_{3}}\], a yellow solid, is one of the two commercially produced phosphorus sulphides. It is a major component of the strike-anywhere matches. Depending on purity of the compound obtained samples can appear yellow-green to grey. Phosphorus sesquisulphide, free from yellow and white phosphorus appears as a perfect yellow crystalline solid.
- This compound has a trigonal pyramidal geometry, having a triangular base made with phosphorus at each corner of the triangle. Each of the phosphorus on the triangular is perpendicularly attached to a sulphur atom. All these sulphur atoms are further attached to a single trivalent phosphorus atom as shown in the figure given below. It is a derivative of the tetrahedral phosphorus unit from insertion of sulphur into three P-P bonds. The P-S and P-P bond lengths are $2.090\,\overset{\circ }{\mathop{A}}\,$ and $2.235\,\overset{\circ }{\mathop{A}}\,$ respectively.
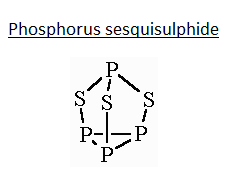
Thus, from the image and explanation given we infer that 6 P-S and 3 P−P bonds are present in the above compound.
Note: \[{{\text{P}}_{4}}{{\text{S}}_{3}}\] compound is made by melting phosphorus and sulphur together at high temperatures and they form the mixed crystals of one dissolved in the other. Excess sulphur gives phosphorus penta-sulphide $({{P}_{4}}{{S}_{10}})$. Its flash point is about ${{100}^{\circ }}C$ .
- It can be easily ignited by friction. It also forms sulphur dioxide and phosphorus penta-oxide during combustion with oxygen. It reacts with water to form phosphoric acid which is a corrosive material. It is also used to make matches and in the manufacture of other chemicals.
Complete Solution :
\[{{\text{P}}_{4}}{{\text{S}}_{3}}\], a yellow solid, is one of the two commercially produced phosphorus sulphides. It is a major component of the strike-anywhere matches. Depending on purity of the compound obtained samples can appear yellow-green to grey. Phosphorus sesquisulphide, free from yellow and white phosphorus appears as a perfect yellow crystalline solid.
- This compound has a trigonal pyramidal geometry, having a triangular base made with phosphorus at each corner of the triangle. Each of the phosphorus on the triangular is perpendicularly attached to a sulphur atom. All these sulphur atoms are further attached to a single trivalent phosphorus atom as shown in the figure given below. It is a derivative of the tetrahedral phosphorus unit from insertion of sulphur into three P-P bonds. The P-S and P-P bond lengths are $2.090\,\overset{\circ }{\mathop{A}}\,$ and $2.235\,\overset{\circ }{\mathop{A}}\,$ respectively.

Thus, from the image and explanation given we infer that 6 P-S and 3 P−P bonds are present in the above compound.
Note: \[{{\text{P}}_{4}}{{\text{S}}_{3}}\] compound is made by melting phosphorus and sulphur together at high temperatures and they form the mixed crystals of one dissolved in the other. Excess sulphur gives phosphorus penta-sulphide $({{P}_{4}}{{S}_{10}})$. Its flash point is about ${{100}^{\circ }}C$ .
- It can be easily ignited by friction. It also forms sulphur dioxide and phosphorus penta-oxide during combustion with oxygen. It reacts with water to form phosphoric acid which is a corrosive material. It is also used to make matches and in the manufacture of other chemicals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




