
Oxidation numbers of chlorine atoms in $CaOC{l_2}$ are
A. 0,0
B. -1, -1
C. -1, +1
D. none of these
Answer
580.8k+ views
Hint: In bleaching powder $CaOC{l_2}$, two chlorine atoms are present. One chlorine atom is attached to the calcium atom directly and other chlorine is attached to the oxygen atom.
Complete step by step answer:
The oxidation number is also known as the oxidation state. It is defined as the overall electron in which an atom either accepts or donates to the neighboring atom to generate a chemical bond with the surrounding atom.
The compound $CaOC{l_2}$ is commonly known as bleaching powder, the chemical name is calcium hypochlorite and is written as Ca(OCl)Cl in which two chlorine atoms with different oxidation states are attached.
The structure of $CaOC{l_2}$ is shown below
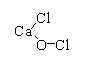
The Ca(OCl)Cl can be written as $[C{a^{ + 2}}{(OCl)^{ - 1}}C{l^{ - 1}}]$
The oxidation state of calcium is +2.
The oxidation state of chlorine attached to calcium is -1.
As we know the oxidation state of oxygen is -2 and the oxidation state of the overall molecule in (OCl) is -1, so the resulting oxidation state of chlorine will be +1. The sum of all the charges should be equal to zero as the compound is neutral.
Thus, the oxidation number of chlorine atoms in $CaOC{l_2}$ is -1 and +1.
Thus, the correct answer is option C.
Note:
The sum of the oxidation state in the polyatomic ion is the same as the charge on the ion but in the compound, $CaOC{l_2}$ no charge is present as it is a neutral compound.
Complete step by step answer:
The oxidation number is also known as the oxidation state. It is defined as the overall electron in which an atom either accepts or donates to the neighboring atom to generate a chemical bond with the surrounding atom.
The compound $CaOC{l_2}$ is commonly known as bleaching powder, the chemical name is calcium hypochlorite and is written as Ca(OCl)Cl in which two chlorine atoms with different oxidation states are attached.
The structure of $CaOC{l_2}$ is shown below

The Ca(OCl)Cl can be written as $[C{a^{ + 2}}{(OCl)^{ - 1}}C{l^{ - 1}}]$
The oxidation state of calcium is +2.
The oxidation state of chlorine attached to calcium is -1.
As we know the oxidation state of oxygen is -2 and the oxidation state of the overall molecule in (OCl) is -1, so the resulting oxidation state of chlorine will be +1. The sum of all the charges should be equal to zero as the compound is neutral.
Thus, the oxidation number of chlorine atoms in $CaOC{l_2}$ is -1 and +1.
Thus, the correct answer is option C.
Note:
The sum of the oxidation state in the polyatomic ion is the same as the charge on the ion but in the compound, $CaOC{l_2}$ no charge is present as it is a neutral compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




