
Oxidation number of sulphur in peroxymonosulfuric acid $\left( {{H_2}S{O_5}} \right)$ is:
$\left( A \right)$ $ + 4$
$\left( B \right)$ $ + 2$
$\left( C \right)$ $ + 6$
$\left( D \right)$ $ - 2$
Answer
570.9k+ views
Hint: Oxidation number is defined as the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. Oxidation number is also known as Oxidation state.
Complete step by step answer:
The peroxymonosulfuric acid is peroxy acid of sulphur. A peroxo acid is formed by peroxo linkage. Peroxo linkage is defined as the in a compound there should bond between oxygen-oxygen bonds present in the compound. If there is The bond is present and it is called peroxy linkage. In ${H_2}S{O_5}$ sulphur atom is bonded to three oxygen atoms and one hydroxyl group. Out of three oxygen atoms one is bonded with a hydroxyl group. This acid is also known as Caro’s acid. We can see the structure of peroxymonosulfuric acid.
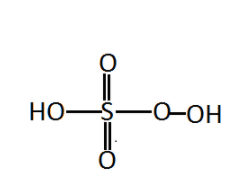
Now, let us find the oxidation state of sulphur in ${H_2}S{O_5}$ , our aim to find the oxidation number of sulphur. So let the oxidation state of sulphur is $X$ .
In peroxymonosulfuric acid there are two peroxide molecules present and the other entire oxygen atom is normally attached. For normal oxygen atom attached to sulphur atom has $ - 2$ charge whereas peroxide molecules contain $ - 1$ charge and the charge on hydrogen atom is $ + 1.$
Then, the oxidation state of sulphur, we get
$2 \times 1 + X + 2 \times \left( { - 1} \right) + 3 \times \left( { - 2} \right) = 0$
$ \Rightarrow $ $X = 6$
Thus, the oxidation state of sulphur in peroxymonosulfuric acid $\left( {{H_2}S{O_5}} \right)$ is $ + 6$ .
So, the correct option is $C.$
Note:
In $\left( {{H_2}S{O_5}} \right)$ there is peroxy linkage shown by two atoms, and therefore in such cases we have to consider the charge of two peroxide molecules and then find the oxidation number or oxidation state of the central atom otherwise we will get wrong answer. In this case the wrong oxidation number of sulphur is $8$ , if we didn’t consider the charge of peroxide molecules separately.
Complete step by step answer:
The peroxymonosulfuric acid is peroxy acid of sulphur. A peroxo acid is formed by peroxo linkage. Peroxo linkage is defined as the in a compound there should bond between oxygen-oxygen bonds present in the compound. If there is The bond is present and it is called peroxy linkage. In ${H_2}S{O_5}$ sulphur atom is bonded to three oxygen atoms and one hydroxyl group. Out of three oxygen atoms one is bonded with a hydroxyl group. This acid is also known as Caro’s acid. We can see the structure of peroxymonosulfuric acid.

Now, let us find the oxidation state of sulphur in ${H_2}S{O_5}$ , our aim to find the oxidation number of sulphur. So let the oxidation state of sulphur is $X$ .
In peroxymonosulfuric acid there are two peroxide molecules present and the other entire oxygen atom is normally attached. For normal oxygen atom attached to sulphur atom has $ - 2$ charge whereas peroxide molecules contain $ - 1$ charge and the charge on hydrogen atom is $ + 1.$
Then, the oxidation state of sulphur, we get
$2 \times 1 + X + 2 \times \left( { - 1} \right) + 3 \times \left( { - 2} \right) = 0$
$ \Rightarrow $ $X = 6$
Thus, the oxidation state of sulphur in peroxymonosulfuric acid $\left( {{H_2}S{O_5}} \right)$ is $ + 6$ .
So, the correct option is $C.$
Note:
In $\left( {{H_2}S{O_5}} \right)$ there is peroxy linkage shown by two atoms, and therefore in such cases we have to consider the charge of two peroxide molecules and then find the oxidation number or oxidation state of the central atom otherwise we will get wrong answer. In this case the wrong oxidation number of sulphur is $8$ , if we didn’t consider the charge of peroxide molecules separately.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




