
What is the oxidation number of $Cr$ in \[{(N{H_3})_3}Cr{O_4}\]or \[{(N{H_3})_3}Cr{({O_2})_2}\]?
Answer
570.3k+ views
Hint: Oxidation Number of an element or compound will depend on the number of electrons that are gained or lost by an atom that is present in a compound. Oxidation number is also known as the Oxidation state of a compound. We can determine the Oxidation number of the element by following certain rules.
Complete step by step solution:
Let us consider of the compound \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\]
The structure of \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\] is a pentagonal bipyramidal. The structure is given below.
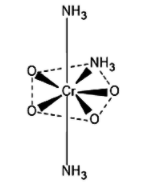
Let us now calculate the oxidation number of $Cr$ in \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\].
Consider the oxidation number of Chromium as \[x\]
The total oxidation number of the compound is 0.
The oxidation number of the \[N{H_3}\] group is 0.
Therefore, \[3 \times 0 = 0\]
The oxidation number of Oxygen is -1. Because the oxygen present in \[{(N{H_3})_3}Cr{O_4}\] is in the peroxide group.
Therefore,
\[3 \times 0 + x + 4 \times - 1 = 0\]
\[x - 4 = 0\]
\[x = 4\]
Therefore, the oxidation number of Cr in \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\] is +4.
Additional information:
We have to follow certain rules to assign the oxidation number to certain elements.
- Atoms that are in the free elemental state i.e. \[({H_2},{O_2})\] will be 0.
- Monatomic ions \[N{a^ + } = 1\]
- Halogen like \[C{l^ - } = - 1\]
- Oxygen =-2
- Hydrogen =1
- Metal hydrides -1.
Note: We must always remember that the oxidation state of the oxygen atom in peroxide will always be -1, as the oxygen atom is linked to only the chromium atom through a single bond. The Oxygen which does not belong to the peroxide molecule will be having the oxidation number of -2.
Complete step by step solution:
Let us consider of the compound \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\]
The structure of \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\] is a pentagonal bipyramidal. The structure is given below.

Let us now calculate the oxidation number of $Cr$ in \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\].
Consider the oxidation number of Chromium as \[x\]
The total oxidation number of the compound is 0.
The oxidation number of the \[N{H_3}\] group is 0.
Therefore, \[3 \times 0 = 0\]
The oxidation number of Oxygen is -1. Because the oxygen present in \[{(N{H_3})_3}Cr{O_4}\] is in the peroxide group.
Therefore,
\[3 \times 0 + x + 4 \times - 1 = 0\]
\[x - 4 = 0\]
\[x = 4\]
Therefore, the oxidation number of Cr in \[{(N{H_3})_3}Cr{O_4}\] or \[{(N{H_3})_3}Cr{({O_2})_2}\] is +4.
Additional information:
We have to follow certain rules to assign the oxidation number to certain elements.
- Atoms that are in the free elemental state i.e. \[({H_2},{O_2})\] will be 0.
- Monatomic ions \[N{a^ + } = 1\]
- Halogen like \[C{l^ - } = - 1\]
- Oxygen =-2
- Hydrogen =1
- Metal hydrides -1.
Note: We must always remember that the oxidation state of the oxygen atom in peroxide will always be -1, as the oxygen atom is linked to only the chromium atom through a single bond. The Oxygen which does not belong to the peroxide molecule will be having the oxidation number of -2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




