
Out of white phosphorous and red phosphorous, which one is more reactive and why?
Answer
560.7k+ views
Hint: The element phosphorous majorly exists in two forms, white phosphorous and red phosphorous. In order to answer this question, we have to first understand the structures of both white phosphorus and red phosphorus.
Complete answer:
White phosphorus and red phosphorus are known as the allotropic forms of phosphorus. Allotropes are compounds that have different structures of the same element. Let us first understand the structures of white phosphorus and red phosphorus individually. The molecular formula of white phosphorus is and each phosphorus atom has hybridization.
We can draw the structure for this white phosphorus as follows:

This is a rectangular tetrahedron and the angle between the bonds is .
Similarly, let us understand the structure of red phosphorus.
The molecular formula of red phosphorus is also but red phosphorus exists in polymer form. So the bond between two phosphorus is broken and a chain of molecules are bonded instead. Let us write the structure for this compound.
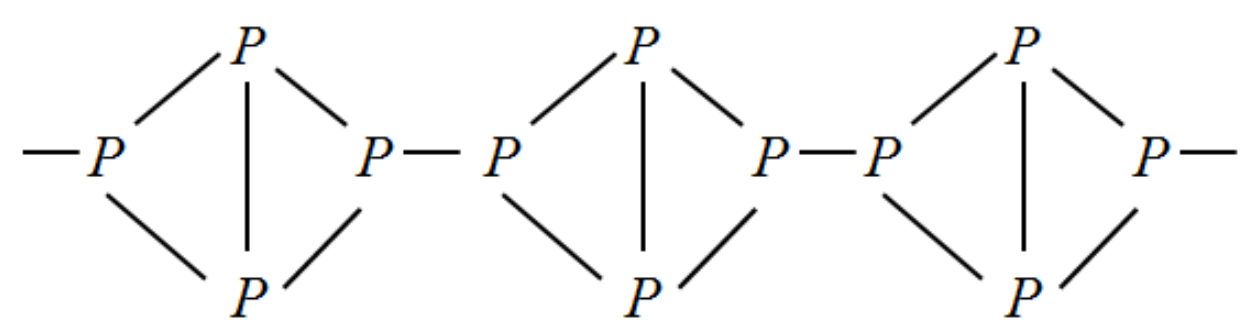
The cross linking in red phosphorus gives it much more stability than white phosphorus. This means that red phosphorus is less reactive when compared to white phosphorus.
Therefore, we can conclude that white phosphorus is more reactive than red phosphorus.
Note: It is to be noted that in white phosphorus, the bonds are close to each other and due to this close packing they have high strain. This strain makes the compound less stable and highly reactive in nature.
Complete answer:
White phosphorus and red phosphorus are known as the allotropic forms of phosphorus. Allotropes are compounds that have different structures of the same element. Let us first understand the structures of white phosphorus and red phosphorus individually. The molecular formula of white phosphorus is and each phosphorus atom has hybridization.
We can draw the structure for this white phosphorus as follows:

This is a rectangular tetrahedron and the angle between the bonds is .
Similarly, let us understand the structure of red phosphorus.
The molecular formula of red phosphorus is also but red phosphorus exists in polymer form. So the bond between two phosphorus is broken and a chain of molecules are bonded instead. Let us write the structure for this compound.

The cross linking in red phosphorus gives it much more stability than white phosphorus. This means that red phosphorus is less reactive when compared to white phosphorus.
Therefore, we can conclude that white phosphorus is more reactive than red phosphorus.
Note: It is to be noted that in white phosphorus, the bonds are close to each other and due to this close packing they have high strain. This strain makes the compound less stable and highly reactive in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




