
What is the order of \[{K_a}\] of the following compounds?
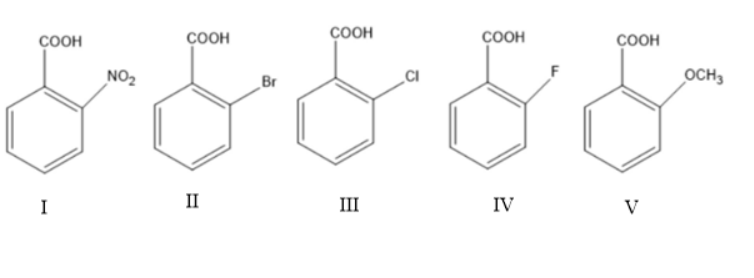
A.\[I > II > III > IV > V\]
B.\[II > I > III > IV > V\]
C.\[V > IV > III > I > II\]
D.\[III > II > I > V > IV\]

Answer
498.9k+ views
Hint: The acids are the compounds that can easily lose \[\left[ {{H^ + }} \right]\] ion or proton. The acid dissociation constant is the value given for acids that gives the idea about the property of acid. Higher the value of \[{K_a}\] stronger is the acid and \[{K_a}\] is inversely proportional to \[p{K_a}\]. Thus, lower is the \[p{K_a}\] stronger is the acid.
Complete answer: The compounds with the presence of \[ - COOH\] can be known as carboxylic acids. Carboxylic acids can easily lose or dissociate to give \[\left[ {{H^ + }} \right]\] ion or proton. The stronger the acid, the easier the dissociation of \[\left[ {{H^ + }} \right]\].
Every acid has dissociation constant value which can be represented as \[{K_a}\]. This acid dissociation constant is inversely proportional to \[p{K_a}\] . Thus, lower is the \[p{K_a}\] of the compound, higher will be the \[{K_a}\] and stronger is the acid.
In the given compounds, nitro benzoic acid has lower \[p{K_a}\] which results in higher \[{K_a}\] and is the strongest acid.
The \[p{K_a}\] order of the remaining compounds will be as follows:
Bromo benzoic acid (II) < Chloro benzoic acid (III) < Fluoro benzoic acid (IV) < Methoxy benzoic acid (V).
Thus, the order of \[{K_a}\] will be opposite to the order of \[p{K_a}\].
Bromo benzoic acid > Chloro benzoic acid > Fluoro benzoic acid > Methoxy benzoic acid.
Thus, the order of \[{K_a}\] is \[I > II > III > IV > V\].
Hence the correct answer is option A.
Note:
Nitro group is an electron withdrawing group and tries to attract electrons from an aromatic ring. Carboxylic acid group on the aromatic ring is also an electron attracting group. Thus, it is stronger than the remaining compounds. In halogens, the acid follows the order based on \[p{K_a}\].
Complete answer: The compounds with the presence of \[ - COOH\] can be known as carboxylic acids. Carboxylic acids can easily lose or dissociate to give \[\left[ {{H^ + }} \right]\] ion or proton. The stronger the acid, the easier the dissociation of \[\left[ {{H^ + }} \right]\].
Every acid has dissociation constant value which can be represented as \[{K_a}\]. This acid dissociation constant is inversely proportional to \[p{K_a}\] . Thus, lower is the \[p{K_a}\] of the compound, higher will be the \[{K_a}\] and stronger is the acid.
In the given compounds, nitro benzoic acid has lower \[p{K_a}\] which results in higher \[{K_a}\] and is the strongest acid.
The \[p{K_a}\] order of the remaining compounds will be as follows:
Bromo benzoic acid (II) < Chloro benzoic acid (III) < Fluoro benzoic acid (IV) < Methoxy benzoic acid (V).
Thus, the order of \[{K_a}\] will be opposite to the order of \[p{K_a}\].
Bromo benzoic acid > Chloro benzoic acid > Fluoro benzoic acid > Methoxy benzoic acid.
Thus, the order of \[{K_a}\] is \[I > II > III > IV > V\].
Hence the correct answer is option A.
Note:
Nitro group is an electron withdrawing group and tries to attract electrons from an aromatic ring. Carboxylic acid group on the aromatic ring is also an electron attracting group. Thus, it is stronger than the remaining compounds. In halogens, the acid follows the order based on \[p{K_a}\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




