
Order of basic strength:
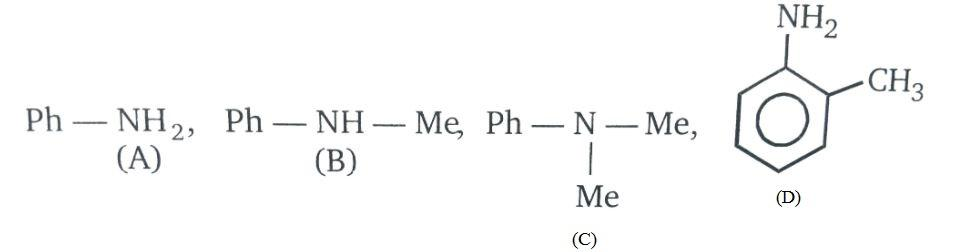
(A) A > B > C > D
(B) B > A > C > D
(C) C > B > A > D
(D) C > B > D > A
Answer
568.8k+ views
Hint: A rough observation tells us that all the given compounds are the nitrogen containing compounds. Every nitrogen containing compound i.e. amines, amides, nitriles, etc., can be compared with respect to the order of basicity.
Complete Solution :
Let us study the factors used to properly determine the basicity of the above given compounds;
The basicity of amines depends on the following factors-
1. The electronic properties of the substituents i.e. the density of electrons on nitrogen atoms.
2. The degree of solvation of the protonated amine i.e. steric hindrance by the groups present on the nitrogen.
- Now, moving towards the illustration,
Basic strength in amines is increased with the increase in electron density on nitrogen atoms i.e. presence of methyl group increases the electron density due to +R effect. Also, due to steric hindrances the ortho substituted aniline will be less basic than aniline.
Therefore, the order of basic strength will be C > B > A > D.
So, the correct answer is “Option C”.
Note: Do note that in general, basicity of an amine is increased by the presence of electron donating groups and is decreased by the presence of electron withdrawing groups.
Complete Solution :
Let us study the factors used to properly determine the basicity of the above given compounds;
The basicity of amines depends on the following factors-
1. The electronic properties of the substituents i.e. the density of electrons on nitrogen atoms.
2. The degree of solvation of the protonated amine i.e. steric hindrance by the groups present on the nitrogen.
- Now, moving towards the illustration,
Basic strength in amines is increased with the increase in electron density on nitrogen atoms i.e. presence of methyl group increases the electron density due to +R effect. Also, due to steric hindrances the ortho substituted aniline will be less basic than aniline.
Therefore, the order of basic strength will be C > B > A > D.
So, the correct answer is “Option C”.
Note: Do note that in general, basicity of an amine is increased by the presence of electron donating groups and is decreased by the presence of electron withdrawing groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




