
One mole of glycerol is treated with an excess of \[\text{HI}\] to obtain isopropyl iodide. The number of \[\text{HI}\] consumed by one mole of glycerol in this reaction is:
Answer
576k+ views
Hint: Hydro iodic acid, \[\text{HI}\], is an oxidising agent that reduces glycerol to 1,2,3-tri iodopropane is formed at first which is further reduced to form 1,2-diiodopropane and again reduced to form 2-iodopropane or isopropyl iodide.
Complete step by step answer:
The reactions can be represented by the following diagram:
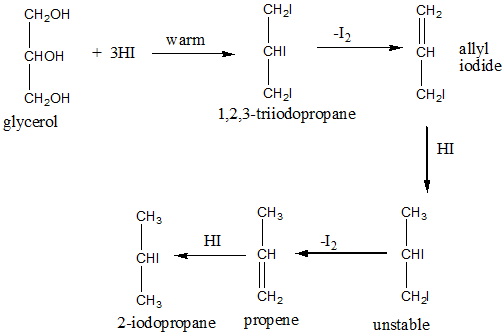
From the above figure it can be seen that a total of 5 moles of Hydrogen Iodide are consumed by 1 mole of glycerol. Three moles of Hydrogen Iodide are consumed in the first reaction when initially 1,2,3-tri iodopropane, which is not much stable and disintegrates quickly to form allyl iodide releasing iodine molecule.
This allyl iodide is further reduced by \[\text{HI}\] to form 1,2-diiodopropane, which is again unstable and dissociates to form propene. Propene is again reduced by \[\text{HI}\] to form 2-iodopropane which is the final product. Hence total 5 moles of \[\text{HI}\] required to reduce 1 mole of glycerol.
Note:
The bond between carbon and iodine is a weak bond due to the difference in their sizes. Hence it dissociates readily to form a stronger bond. The reaction between Glycerol and Hydrogen Iodide takes place in two ways, firstly, when glycerol is warmed with a small amount of Hydrogen Iodide, giving result to allyl iodide and secondly when both glycerol and Hydrogen Iodide are heated, the allyl iodide formed is reduced to propene which is reduced by Hydrogen Iodide again to form 2-propyl iodide or isopropyl iodide.
Complete step by step answer:
The reactions can be represented by the following diagram:

From the above figure it can be seen that a total of 5 moles of Hydrogen Iodide are consumed by 1 mole of glycerol. Three moles of Hydrogen Iodide are consumed in the first reaction when initially 1,2,3-tri iodopropane, which is not much stable and disintegrates quickly to form allyl iodide releasing iodine molecule.
This allyl iodide is further reduced by \[\text{HI}\] to form 1,2-diiodopropane, which is again unstable and dissociates to form propene. Propene is again reduced by \[\text{HI}\] to form 2-iodopropane which is the final product. Hence total 5 moles of \[\text{HI}\] required to reduce 1 mole of glycerol.
Note:
The bond between carbon and iodine is a weak bond due to the difference in their sizes. Hence it dissociates readily to form a stronger bond. The reaction between Glycerol and Hydrogen Iodide takes place in two ways, firstly, when glycerol is warmed with a small amount of Hydrogen Iodide, giving result to allyl iodide and secondly when both glycerol and Hydrogen Iodide are heated, the allyl iodide formed is reduced to propene which is reduced by Hydrogen Iodide again to form 2-propyl iodide or isopropyl iodide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




