
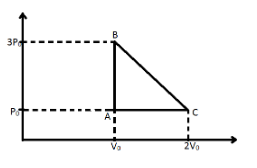
One mole of an ideal monatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by:-
A. $\dfrac{{25}}{8}\dfrac{{{P_0}{V_0}}}{R}$
B. $\dfrac{{25}}{4}\dfrac{{{P_0}{V_0}}}{R}$
C. $\dfrac{{25}}{{16}}\dfrac{{{P_0}{V_0}}}{R}$
D. $\dfrac{5}{8}\dfrac{{{P_0}{V_0}}}{R}$

Answer
573.3k+ views
Hint: An ideal gas equation is given as $PV = nRT$. We can write the PV equation for the process BC using the equation of straight line $y - {y_1} = \dfrac{{{y_2} - {y_1}}}{{{x_2} - {x_1}}} \left( {x - {x_1}} \right)$, where $\left( {{x_1},{y_1}} \right) and \left( {{x_2},{y_2}} \right)$ are known points B and C. We can convert this PV equation in terms of temperature T using the ideal gas equation. For maximum temperature $\dfrac{{dT}}{{dV}} = 0$.
Complete step by step answer:
We know that an ideal gas equation is given as $PV = nRT$. Therefore temperature $T = \dfrac{{PV}}{{nR}}$.
Given, number of moles, n$ = 1$.
Temperature of point A$ = \dfrac{{3{P_0}{V_0}}}{R}$
Temperature off point B$ = \dfrac{{{P_0}2{V_0}}}{R}$
Now we write the PV equation for the process BC using the equation of a straight line with two given point.
$
P - 3{P_0} = \dfrac{{{P_0} - 3{P_0}}}{{2{V_0} - {V_0}}}\left( {V - {V_0}} \right) \\
\Rightarrow P - 3{P_0} = \dfrac{{ - 2{P_0}}}{{{V_0}}}\left( {V - {V_0}} \right) \\
\Rightarrow P = \dfrac{{ - 2{P_0}V}}{{{V_0}}} + 5{P_0} \\ $
Multiplying the equation with $V$.
$PV = \dfrac{{ - 2{P_0}{V^2}}}{{{V_0}}} + 5{P_0}V$
Replacing $PV$ with $RT$.
$RT = - \dfrac{{2{P_0}}}{{{V_0}}}{V^2} + 5{P_0}V$
For maximum temperature $\dfrac{{dT}}{{dV}} = 0$.
Therefore we get the maximum temperature t as
$\therefore T =\dfrac{{25}}{8}\dfrac{{{P_0}{V_0}}}{R}$
Note: It is important to know the equation for ideal gas to solve this question ($PV = nRT$). We find the maximum or minimum value of any quantity using differentiation.For the net work involved in a cyclic process is the area enclosed in a P-V diagram, if the cycle goes clockwise, the system does work and if the cycle goes anticlockwise, then work is done on the system.
Complete step by step answer:
We know that an ideal gas equation is given as $PV = nRT$. Therefore temperature $T = \dfrac{{PV}}{{nR}}$.
Given, number of moles, n$ = 1$.
Temperature of point A$ = \dfrac{{3{P_0}{V_0}}}{R}$
Temperature off point B$ = \dfrac{{{P_0}2{V_0}}}{R}$
Now we write the PV equation for the process BC using the equation of a straight line with two given point.
$
P - 3{P_0} = \dfrac{{{P_0} - 3{P_0}}}{{2{V_0} - {V_0}}}\left( {V - {V_0}} \right) \\
\Rightarrow P - 3{P_0} = \dfrac{{ - 2{P_0}}}{{{V_0}}}\left( {V - {V_0}} \right) \\
\Rightarrow P = \dfrac{{ - 2{P_0}V}}{{{V_0}}} + 5{P_0} \\ $
Multiplying the equation with $V$.
$PV = \dfrac{{ - 2{P_0}{V^2}}}{{{V_0}}} + 5{P_0}V$
Replacing $PV$ with $RT$.
$RT = - \dfrac{{2{P_0}}}{{{V_0}}}{V^2} + 5{P_0}V$
For maximum temperature $\dfrac{{dT}}{{dV}} = 0$.
Therefore we get the maximum temperature t as
$\therefore T =\dfrac{{25}}{8}\dfrac{{{P_0}{V_0}}}{R}$
Note: It is important to know the equation for ideal gas to solve this question ($PV = nRT$). We find the maximum or minimum value of any quantity using differentiation.For the net work involved in a cyclic process is the area enclosed in a P-V diagram, if the cycle goes clockwise, the system does work and if the cycle goes anticlockwise, then work is done on the system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




