
On heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\] gives the gas:
A.\[S{{O}_{2}}\]
B.\[C{{O}_{2}}\]
C.\[CO\]
D.\[N{{O}_{2}}\]
Answer
599.1k+ views
Hint: The gas produced on heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\] has unsaturated carbon-oxygen bond with two pi bonds and one sigma bond. In coordination complexes, it participates in back-bonding with the metal atom and known as carbonyl ligand.
It is very toxic to the human body.
Complete step by step answer:
The gas produced on heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\] is carbon monoxide. Its chemical formula is \[CO\].
The structure of \[CO\]is as follows:
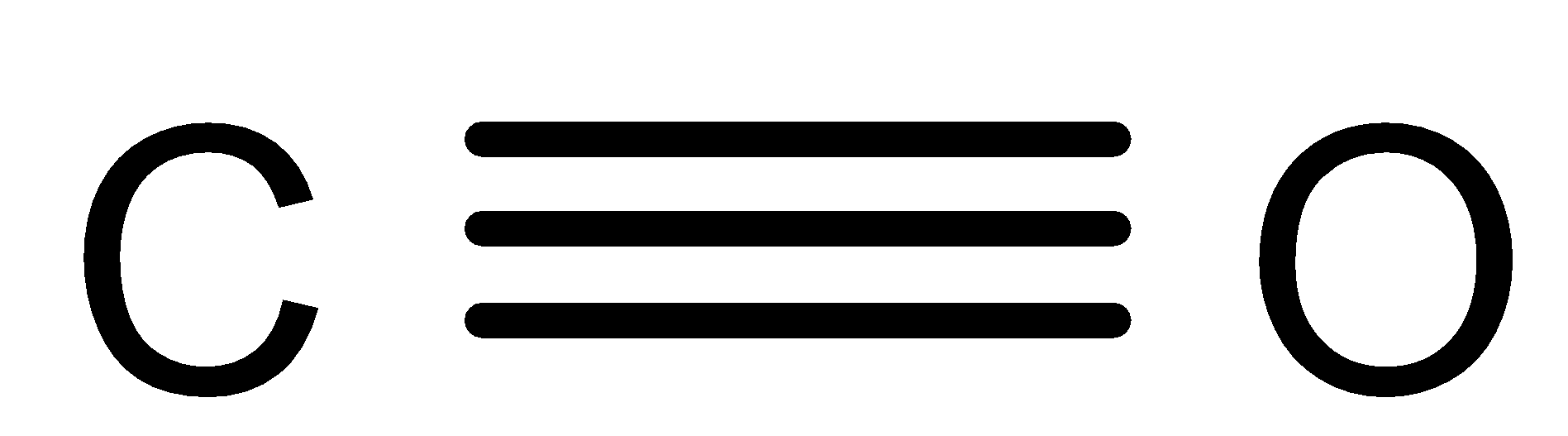
It is produced from the partial oxidation of the carbon-containing compounds.
It is a gas without any colour, taste and odour. It is a flammable gas and produces blue flame on heating. It is slightly lighter than air and is also known as ‘carbonous oxide’.
On reaction with water, \[{{K}_{4}}Fe{{(CN)}_{6}}\]dissociates as:
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\rightleftharpoons \text{ }F{{e}^{+2}}(aq)\text{ }+\text{ }6C{{N}^{-}}(aq)\]
When, \[F{{e}^{+2}}\]reacts with conc.\[{{H}_{2}}S{{O}_{4}}\], it dissociates into \[F{{e}^{+2}}\](aq), HCN and\[S{{O}_{4}}^{2-}\](aq) ions.
Finally, after treating it with the acidic hydrogen followed by dehydration, the products formed are carbon monoxide (\[CO\]), ferrous sulphate (\[FeS{{O}_{4}}\]) and other side products.
The reaction is as follows:
\[{{K}_{4}}\left[ Fe{{\left( CN \right)}_{6}} \right]\text{ }+6{{H}_{2}}S{{O}_{4}}\text{ }+6{{H}_{2}}O\text{ }\to \text{ }6CO\text{ }+\text{ }FeS{{O}_{4}}\text{ }+3\left( N{{H}_{4}} \right)2SO4\text{ }+\text{ }2{{K}_{2}}S{{O}_{4}}\]
So, on heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\], the gas produced is carbon monoxide.
So, the correct option is C.
Note: On heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with dilute\[{{H}_{2}}S{{O}_{4}}\], the gas evolved is hydrogen cyanide (HCN) in the intermediary step.
It is an indirect and weak greenhouse gas. Its emission in the atmosphere causes global warming. When it enters our body, it deprives the vital organs of the body from oxygen and hence causing fatal problems in the human body.
It is very toxic to the human body.
Complete step by step answer:
The gas produced on heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\] is carbon monoxide. Its chemical formula is \[CO\].
The structure of \[CO\]is as follows:
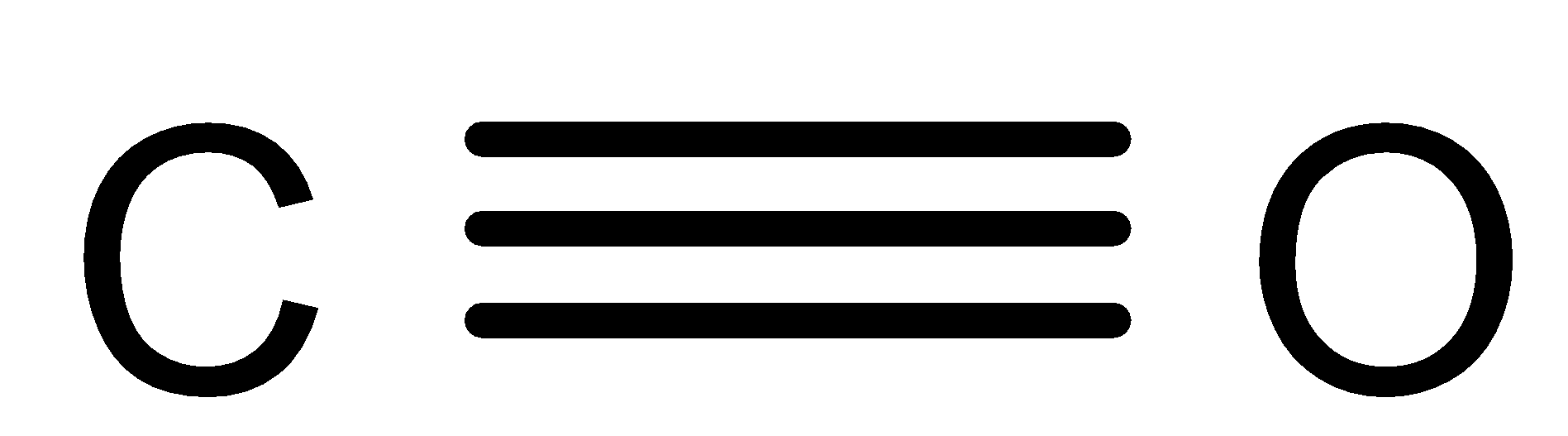
It is produced from the partial oxidation of the carbon-containing compounds.
It is a gas without any colour, taste and odour. It is a flammable gas and produces blue flame on heating. It is slightly lighter than air and is also known as ‘carbonous oxide’.
On reaction with water, \[{{K}_{4}}Fe{{(CN)}_{6}}\]dissociates as:
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\rightleftharpoons \text{ }F{{e}^{+2}}(aq)\text{ }+\text{ }6C{{N}^{-}}(aq)\]
When, \[F{{e}^{+2}}\]reacts with conc.\[{{H}_{2}}S{{O}_{4}}\], it dissociates into \[F{{e}^{+2}}\](aq), HCN and\[S{{O}_{4}}^{2-}\](aq) ions.
Finally, after treating it with the acidic hydrogen followed by dehydration, the products formed are carbon monoxide (\[CO\]), ferrous sulphate (\[FeS{{O}_{4}}\]) and other side products.
The reaction is as follows:
\[{{K}_{4}}\left[ Fe{{\left( CN \right)}_{6}} \right]\text{ }+6{{H}_{2}}S{{O}_{4}}\text{ }+6{{H}_{2}}O\text{ }\to \text{ }6CO\text{ }+\text{ }FeS{{O}_{4}}\text{ }+3\left( N{{H}_{4}} \right)2SO4\text{ }+\text{ }2{{K}_{2}}S{{O}_{4}}\]
So, on heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with conc.\[{{H}_{2}}S{{O}_{4}}\], the gas produced is carbon monoxide.
So, the correct option is C.
Note: On heating \[{{K}_{4}}Fe{{(CN)}_{6}}\] with dilute\[{{H}_{2}}S{{O}_{4}}\], the gas evolved is hydrogen cyanide (HCN) in the intermediary step.
It is an indirect and weak greenhouse gas. Its emission in the atmosphere causes global warming. When it enters our body, it deprives the vital organs of the body from oxygen and hence causing fatal problems in the human body.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




