
On heating glycerol with \[KHS{O_4}/\Delta \], a compound is obtained, which has a bad odour. The compound is:
A. acrolein
B. formic acid
C. allyl alcohol
D. methyl isocyanide
Answer
585.3k+ views
Hint: We can solve this problem by the detailed study of glycerol and its reactions. Glycerol is a simple polyol compound which does not have colour and odour and also it is a viscous liquid which is non-toxic. The reaction with \[KHS{O_4}/\Delta \] forms acrylaldehyde which is also known as acrolein.
Complete step by step answer:
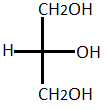
Glycerol is obtained from the animals and plants sources. It is used in the saponification, hydrolysis and transesterification reaction. It has three carbon chains having three hydroxyl groups which provide it hygroscopic nature and allow it to react with many acids to form esters. Glycerol has higher specific gravity than water and also it is easily soluble in water. The basic structure of glycerol is given as follows:
Acrolein is the unsaturated aldehyde also called propenal which is colourless liquid and have bad smell which irritates. The chemical formula for acrolein is ${C_3}{H_4}O$ . It is prepared from the propene. When the propene is oxidized in the air in the presence of catalysts it forms acrolein. But there are also other reactions which form acrolein.
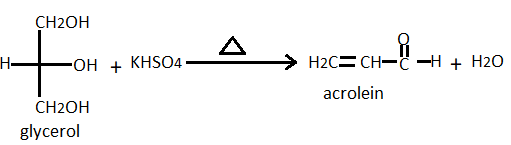
In the given question, when glycerol heats with \[KHS{O_4}/\Delta \], it undergoes dehydration with the removal of two molecules of water from the glycerol, and also results in the formation of unsaturated aldehyde that is also known as acrylic aldehyde. This is the acrolein which is responsible for the bad odour. The reaction is given below;
The compound obtained which has a bad odour is acrolein. So, the correct answer is “Option A”.
Note: In such a type of question we have to check all the options, like formic acid and methyl isocyanide both have a pungent smell and both are not formed from the glycerol. While allyl alcohol has mustard oil smell.
Complete step by step answer:
Glycerol is obtained from the animals and plants sources. It is used in the saponification, hydrolysis and transesterification reaction. It has three carbon chains having three hydroxyl groups which provide it hygroscopic nature and allow it to react with many acids to form esters. Glycerol has higher specific gravity than water and also it is easily soluble in water. The basic structure of glycerol is given as follows:

Acrolein is the unsaturated aldehyde also called propenal which is colourless liquid and have bad smell which irritates. The chemical formula for acrolein is ${C_3}{H_4}O$ . It is prepared from the propene. When the propene is oxidized in the air in the presence of catalysts it forms acrolein. But there are also other reactions which form acrolein.
In the given question, when glycerol heats with \[KHS{O_4}/\Delta \], it undergoes dehydration with the removal of two molecules of water from the glycerol, and also results in the formation of unsaturated aldehyde that is also known as acrylic aldehyde. This is the acrolein which is responsible for the bad odour. The reaction is given below;

The compound obtained which has a bad odour is acrolein. So, the correct answer is “Option A”.
Note: In such a type of question we have to check all the options, like formic acid and methyl isocyanide both have a pungent smell and both are not formed from the glycerol. While allyl alcohol has mustard oil smell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




