
Of the five isomers of hexanes, the isomer which can give two monochlorinated compounds is:
A.2-methyl pentane
B.2,2-dimethylbutane
C.2,3-dimethylbutane
D.n-hexane
Answer
569.7k+ views
Hint: We know that isomers are the compounds which possess the same molecular formula but different atomic arrangement. There are different types of isomers namely, functional isomers, tautomers, constitutional isomers etc.
Complete step by step answer:
Let us first draw all the isomers of ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{14}}}}$.
1.
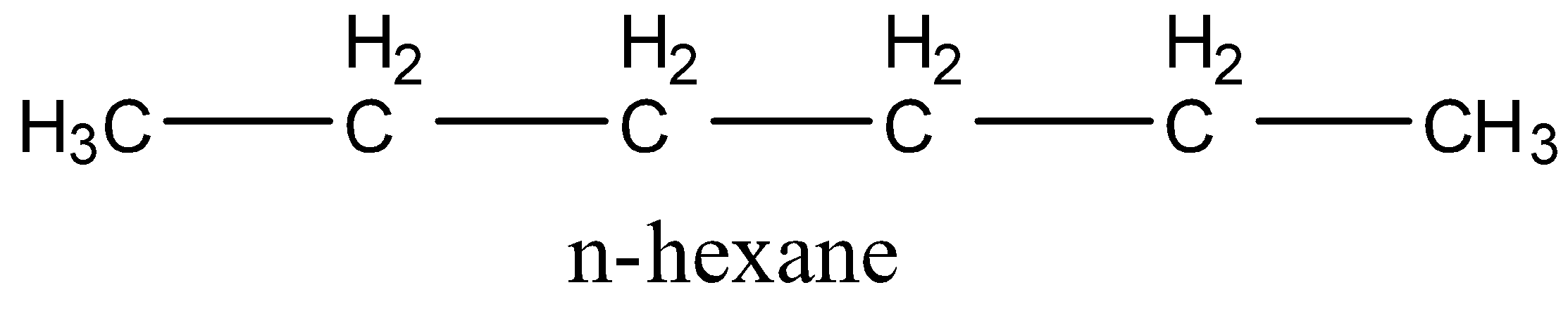
2.
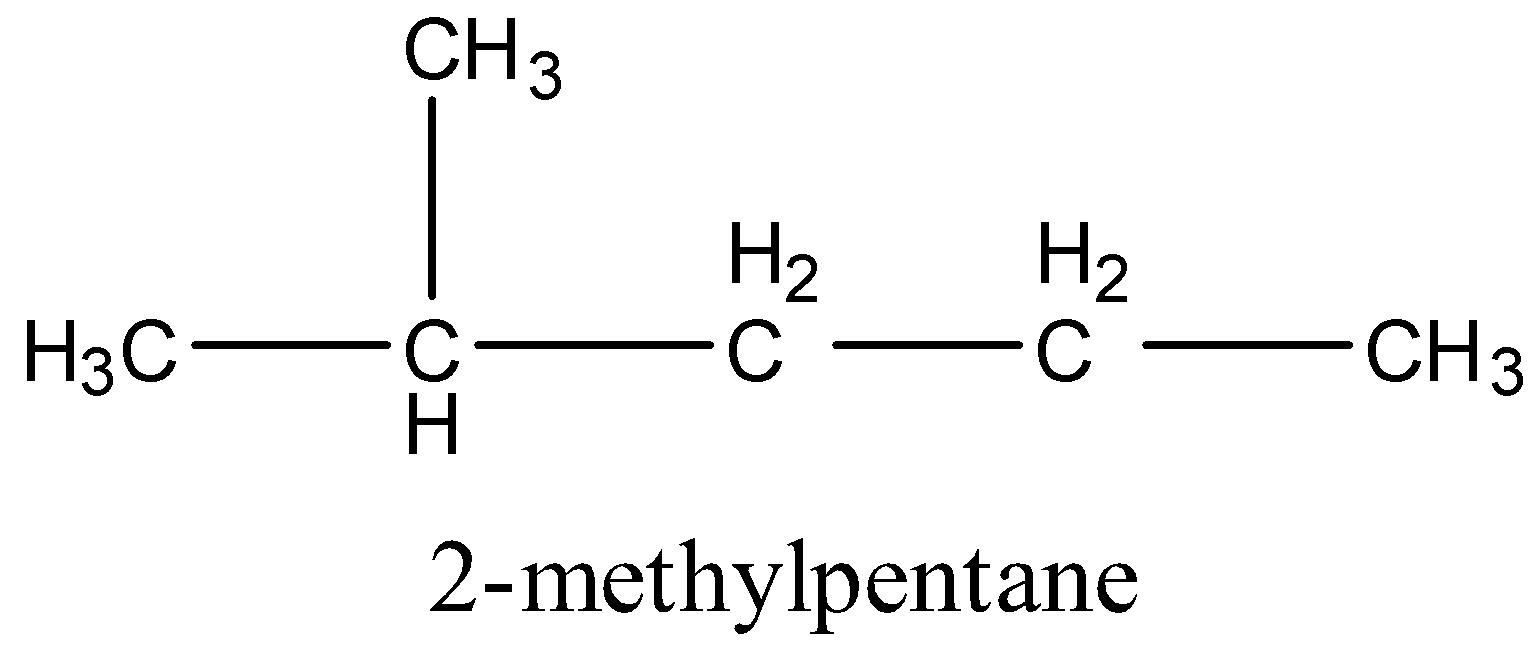
3.
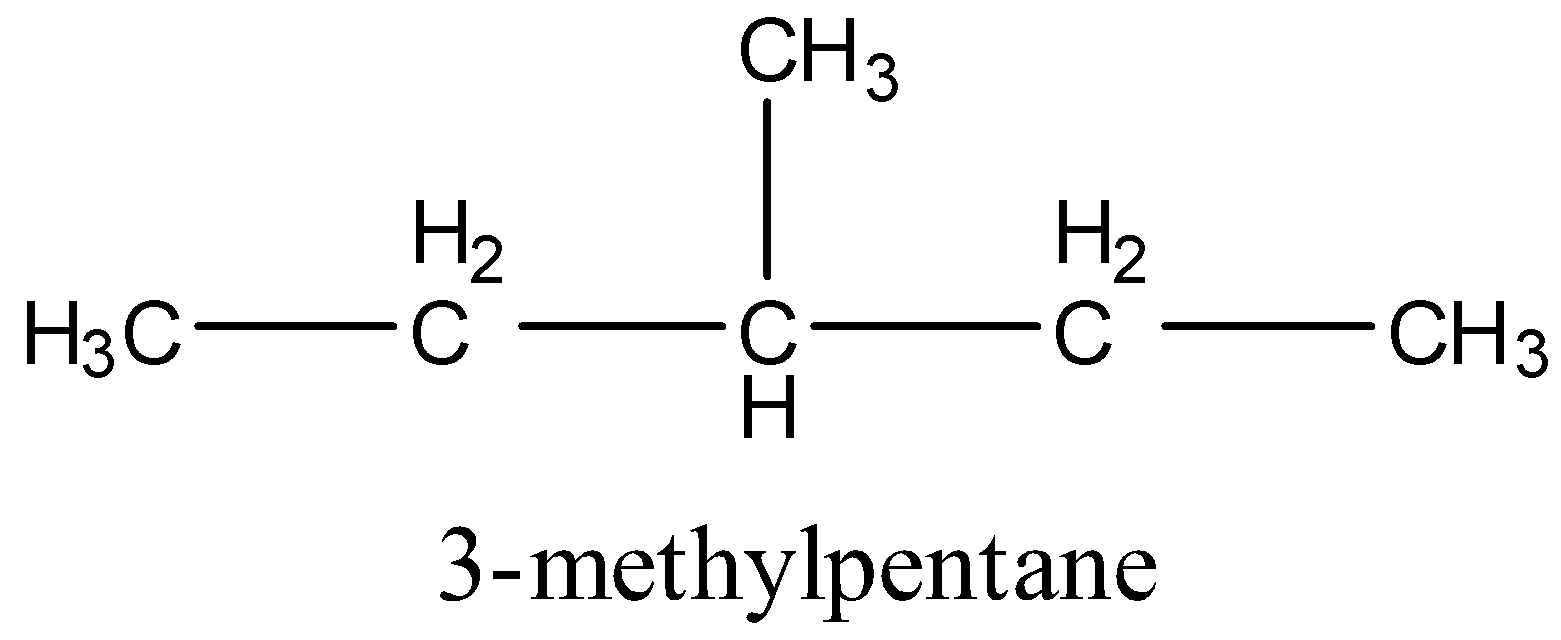
4 .
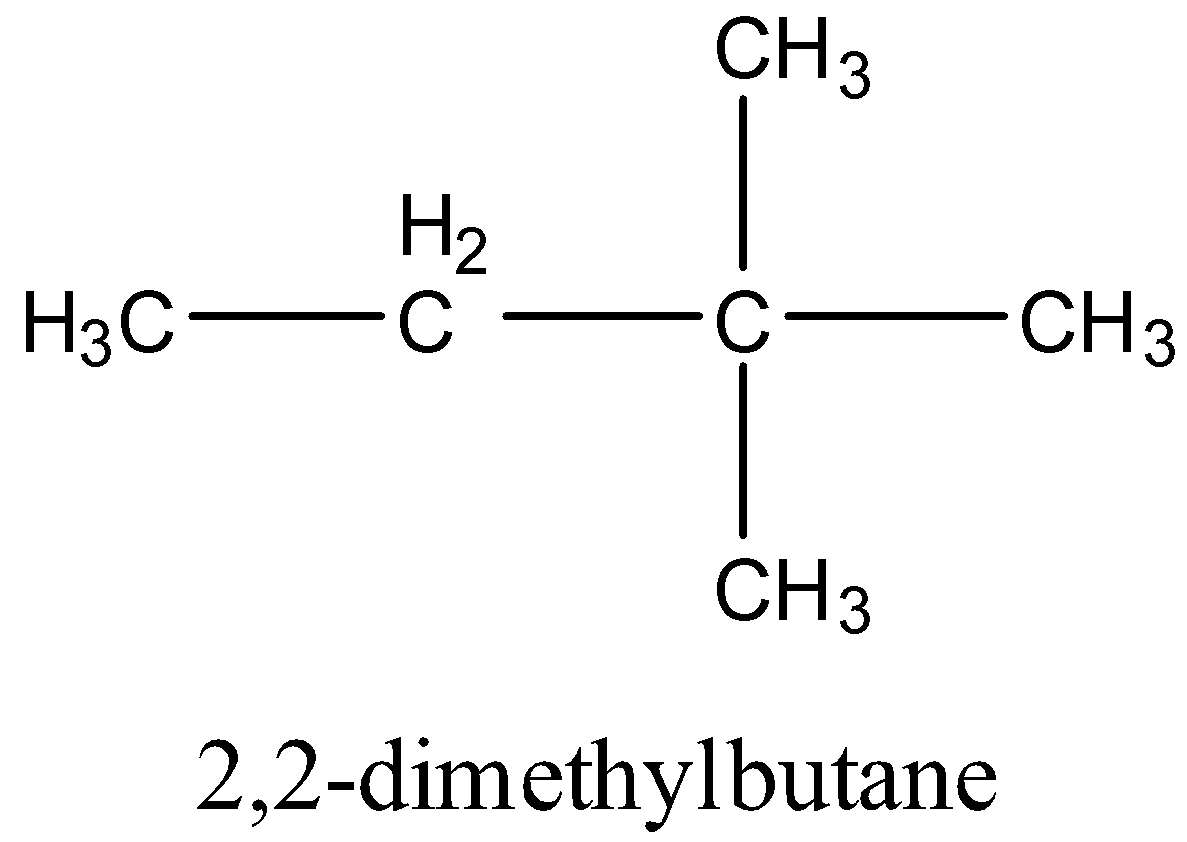
5 .
Let us understand the condition of formation of two monochlorinated products. If the isomeric hexane has two different types of hydrogen and four similar types of hydrogen, then it undergoes reaction with chlorine in presence of light to give two monochlorinated products.
Now, we have to identify the isomer of hexane possessing two different types of H and 4 similar types of H.
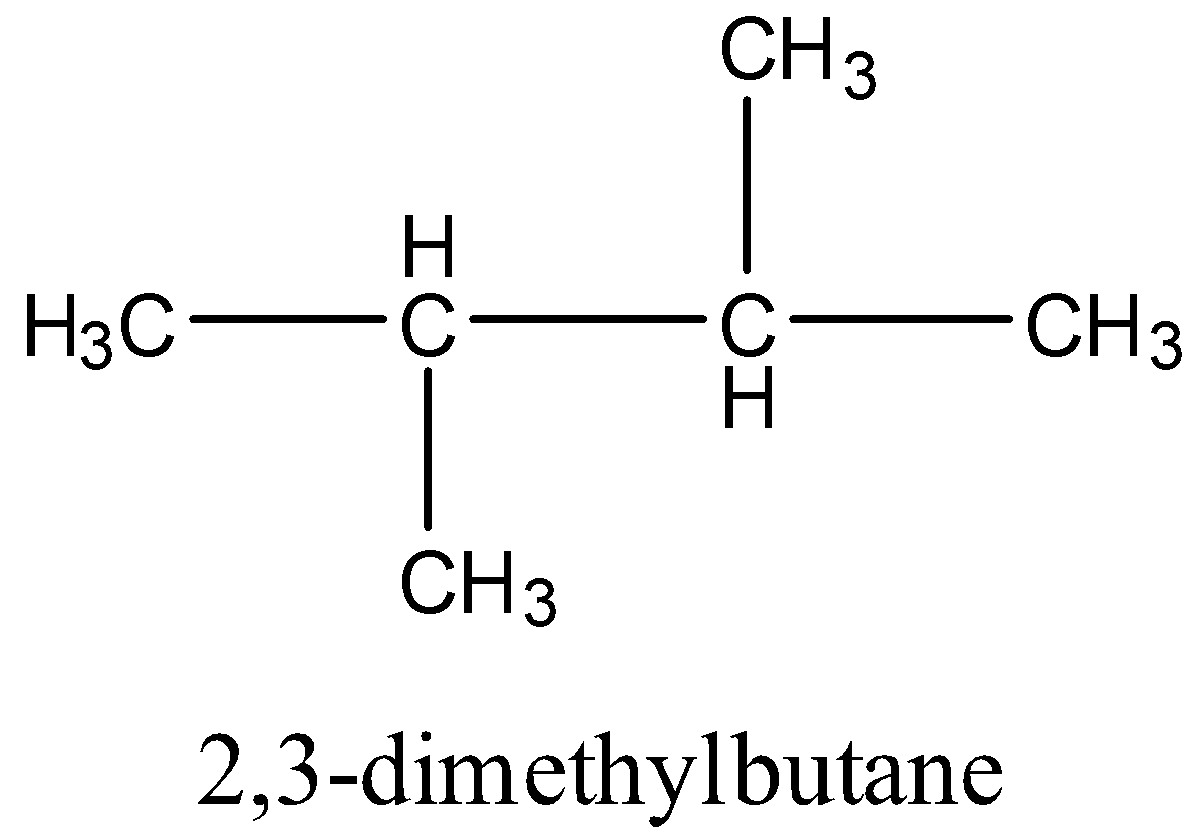
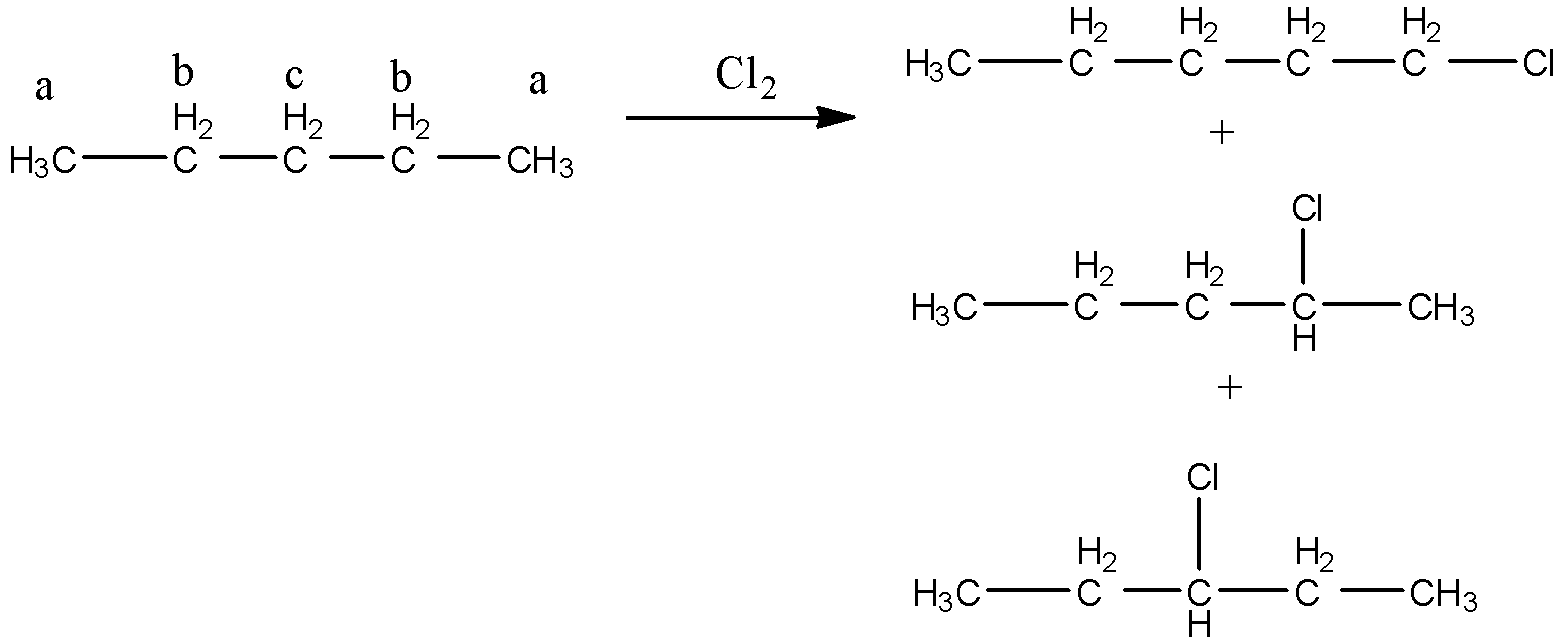
In 2,3-dimethyl butane, 2 types of hydrogen are present.
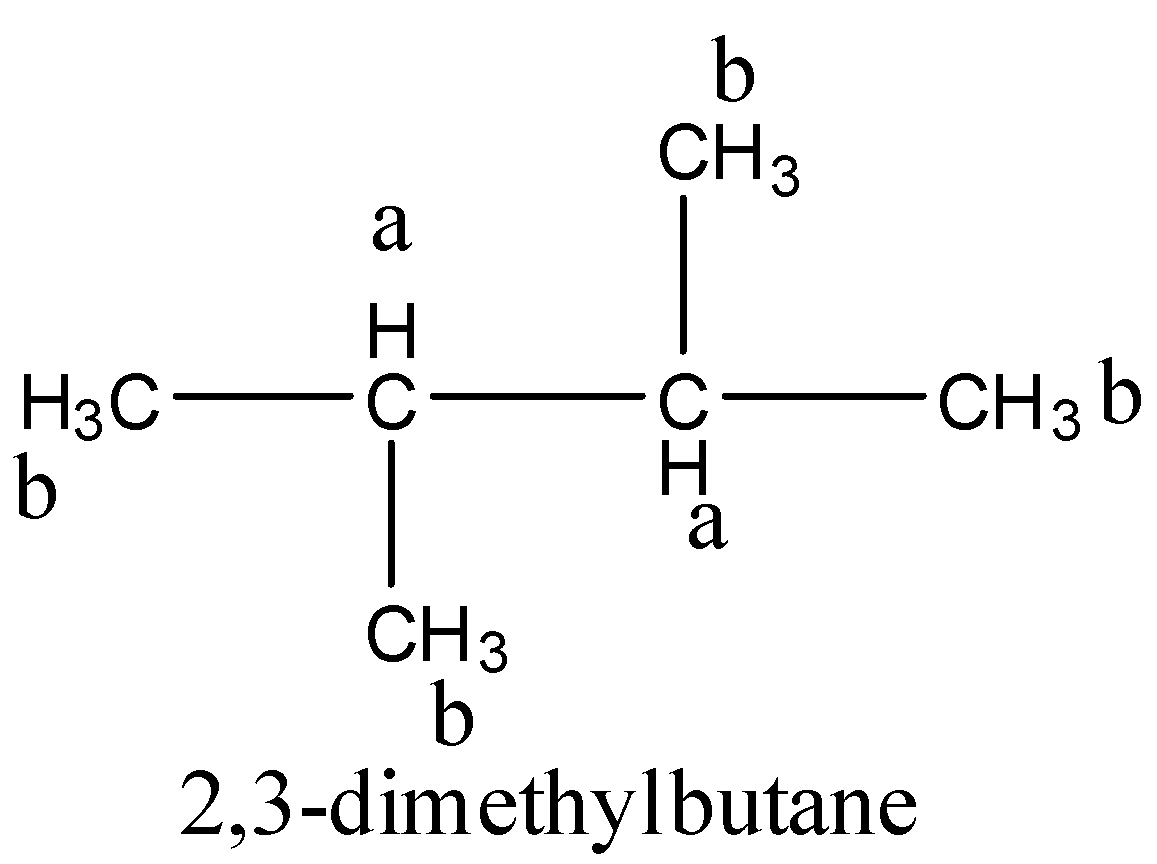
From the above structure, it is clear that there are two different types of hydrogen (a and b) and four similar types hydrogen (a). So, two positions are available for chlorination. Hence,2,3-dimethylbutane gives two monochlorinated products.
The monochlorination reaction is shown as below:
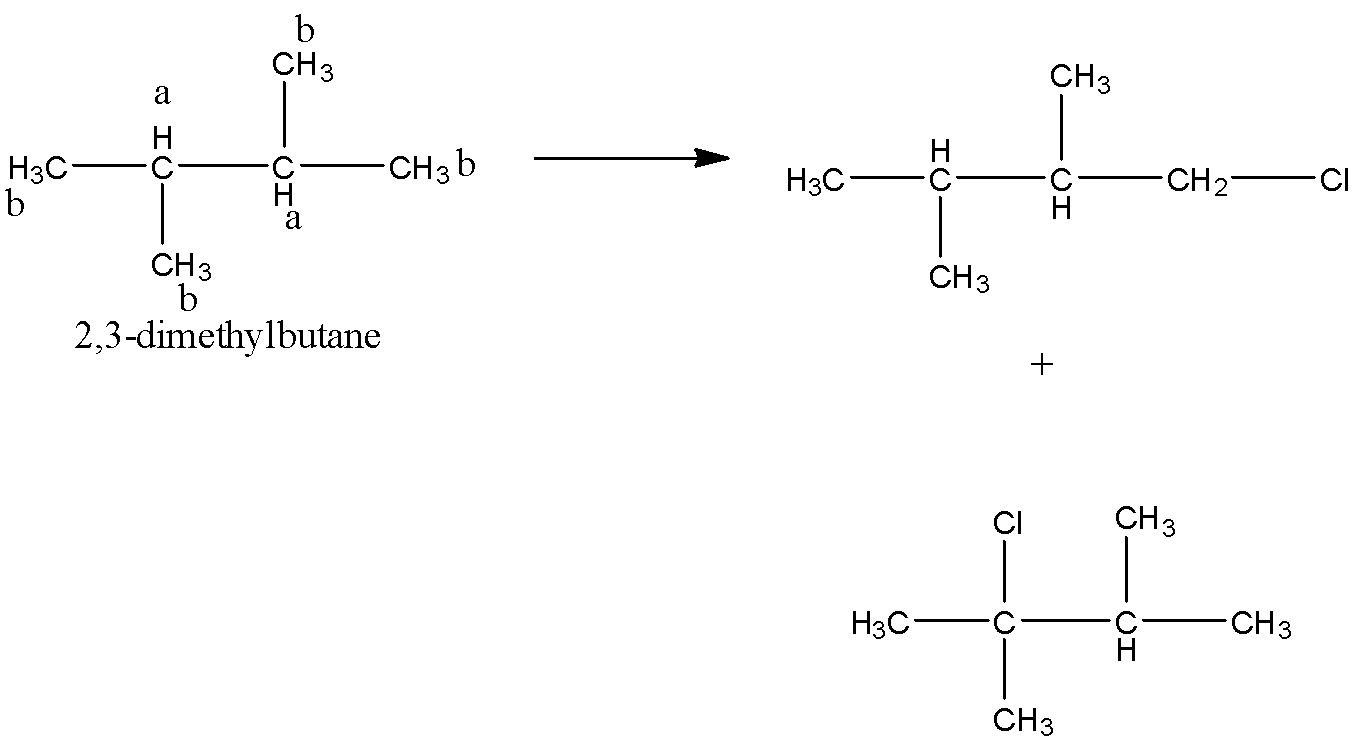
So, out of five isomers of hexane 2,3-dimethylbutane gives monochlorinated products.
So, the correct answer is Option C .
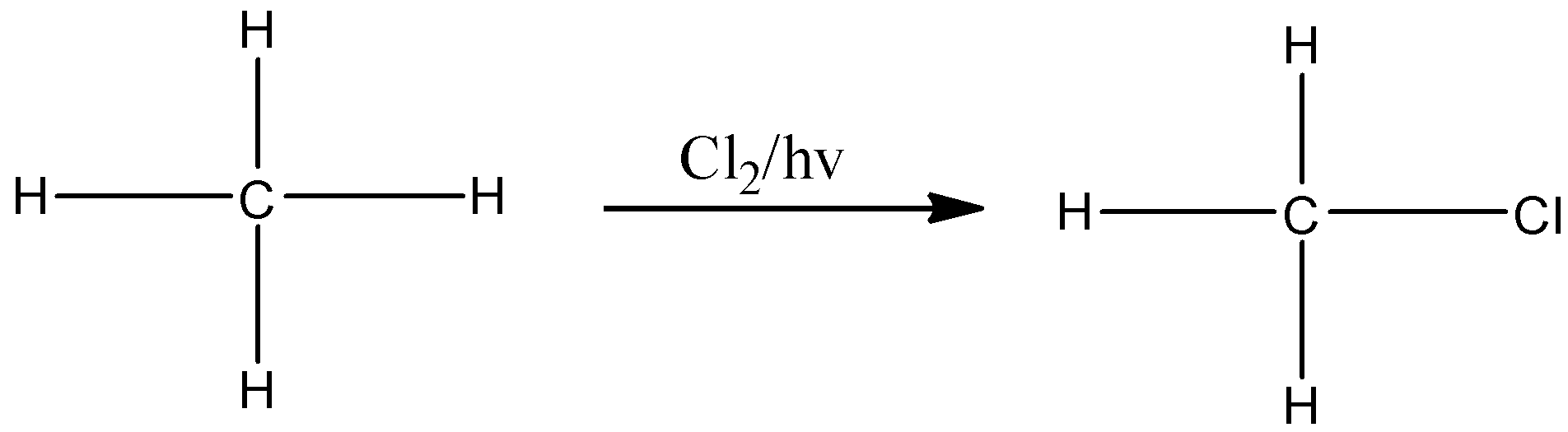
Note: Always remember that the type of hydrogens in a compound decides the number of monochlorinated products. For example, methane has only one type of hydrogen. So, it gives only one monochlorination product, that is,
Pentane is the compound in which three types of hydrogen present. So, it will give three monochlorination products.
Complete step by step answer:
Let us first draw all the isomers of ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{14}}}}$.
1.
2.

3.

4 .

5 .

Let us understand the condition of formation of two monochlorinated products. If the isomeric hexane has two different types of hydrogen and four similar types of hydrogen, then it undergoes reaction with chlorine in presence of light to give two monochlorinated products.
Now, we have to identify the isomer of hexane possessing two different types of H and 4 similar types of H.
In 2,3-dimethyl butane, 2 types of hydrogen are present.

From the above structure, it is clear that there are two different types of hydrogen (a and b) and four similar types hydrogen (a). So, two positions are available for chlorination. Hence,2,3-dimethylbutane gives two monochlorinated products.
The monochlorination reaction is shown as below:

So, out of five isomers of hexane 2,3-dimethylbutane gives monochlorinated products.
So, the correct answer is Option C .
Note: Always remember that the type of hydrogens in a compound decides the number of monochlorinated products. For example, methane has only one type of hydrogen. So, it gives only one monochlorination product, that is,

Pentane is the compound in which three types of hydrogen present. So, it will give three monochlorination products.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




