
Number of unit cells in $4g$ of $X$(atomic mass $ = 40$), which crystallises in the bcc pattern is: (${N_A}$ is Avogadro’s number)
A. $0.1{N_A}$
B. $2 \times 0.1{N_A}$
C. $\dfrac{{0.1 \times {N_A}}}{2}$
D. $2 \times {N_A}$
Answer
497.1k+ views
Hint: In BCC lattice, the atoms are present at each corner and at the centre of the cubic crystal. For the given question, first find the number of atoms in the given mass of $X$ and the number of atoms comprises one unit cell of the bcc lattice. Then by comparing values, we will get the number of unit cells for a given mass of $X$.
Complete answer:
As we know, for BCC lattice, the atoms are present at each corner and at the centre of the cubic crystal as shown in the figure below:
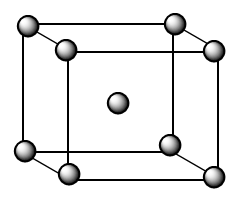
The contribution of each atom present at the corner $ = \dfrac{1}{8}$
The contribution of the atom present at the centre $ = 1$
Therefore, the number of atoms which comprises one unit cell of bcc lattice $ = 8 \times \dfrac{1}{8} + 1 \Rightarrow 2$
Now as per question, the given mass of $X = 4g$
Atomic mass of $X = 40g$
So, the number of moles of $X = \dfrac{{{\text{given mass}}}}{{{\text{atomic mass}}}}$
$ \Rightarrow n = \dfrac{4}{{40}}$
$ \Rightarrow n = 0.1$
Therefore, the number of atoms of $X = n \times {N_A} \Rightarrow 0.1 \times {N_A}$
For BCC unit lattice:
$\because $ 2 atoms comprise to form $ \Rightarrow $1 unit cell
$\therefore 0.1 \times {N_A}$ atoms will comprise to form $ \Rightarrow \dfrac{{0.1 \times {N_A}}}{2}$ unit cells.
Hence for a bcc unit lattice, the number of unit cells in $4g$ of $X$ is $\dfrac{{0.1 \times {N_A}}}{2}$.
So, option (C) is the correct answer.
Note:
Remember that atoms in BCC unit lattice are much more efficiently packed as compared to simple cubic structure, occupying about $68\% $ of the total volume. The coordination number i.e., the number of nearest neighbour atoms in a bcc lattice is eight.
Complete answer:
As we know, for BCC lattice, the atoms are present at each corner and at the centre of the cubic crystal as shown in the figure below:

The contribution of each atom present at the corner $ = \dfrac{1}{8}$
The contribution of the atom present at the centre $ = 1$
Therefore, the number of atoms which comprises one unit cell of bcc lattice $ = 8 \times \dfrac{1}{8} + 1 \Rightarrow 2$
Now as per question, the given mass of $X = 4g$
Atomic mass of $X = 40g$
So, the number of moles of $X = \dfrac{{{\text{given mass}}}}{{{\text{atomic mass}}}}$
$ \Rightarrow n = \dfrac{4}{{40}}$
$ \Rightarrow n = 0.1$
Therefore, the number of atoms of $X = n \times {N_A} \Rightarrow 0.1 \times {N_A}$
For BCC unit lattice:
$\because $ 2 atoms comprise to form $ \Rightarrow $1 unit cell
$\therefore 0.1 \times {N_A}$ atoms will comprise to form $ \Rightarrow \dfrac{{0.1 \times {N_A}}}{2}$ unit cells.
Hence for a bcc unit lattice, the number of unit cells in $4g$ of $X$ is $\dfrac{{0.1 \times {N_A}}}{2}$.
So, option (C) is the correct answer.
Note:
Remember that atoms in BCC unit lattice are much more efficiently packed as compared to simple cubic structure, occupying about $68\% $ of the total volume. The coordination number i.e., the number of nearest neighbour atoms in a bcc lattice is eight.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




