
Number of moles of hydrogen atoms required to get one mole of hydrazobenzene from nitrobenzene is:
A. 10
B. 5
C. 8
D. 4
Answer
568.5k+ views
Hint: Nitrobenzene has the chemical formula of \[{{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{O}}_{2}}\]. Whereas hydrazobenzene has two aromatic rings which are attached with an amine group. A number of moles of a substance are the ratio of the mass to the molecular mass of the compound.
Complete Solution:
-The process of formation of hydrazobenzene includes a total of five steps in which the reduction of every compound takes place.
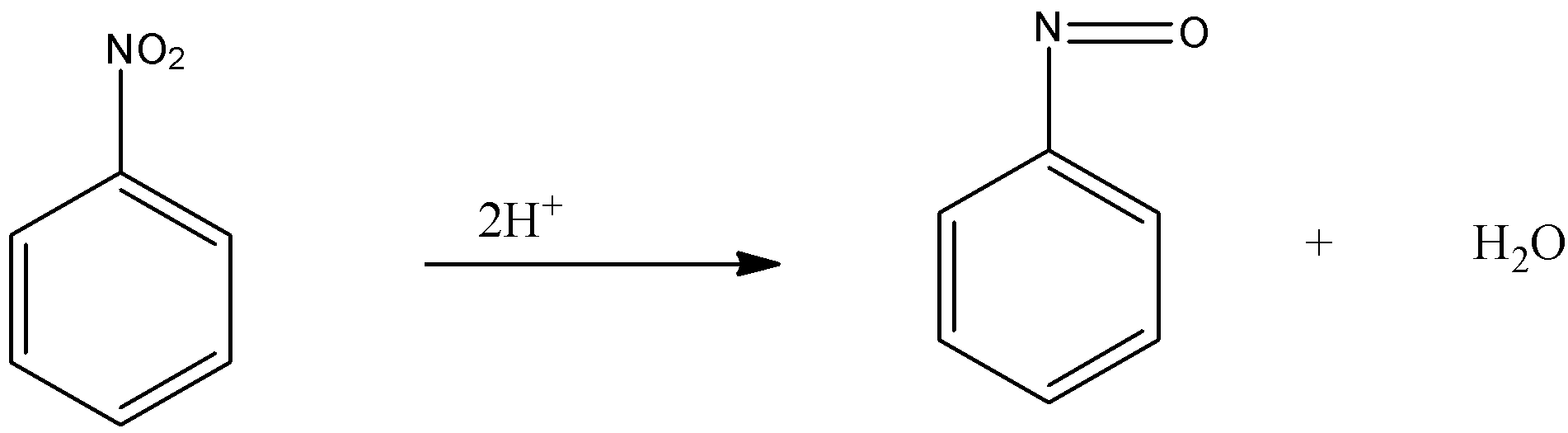
-Firstly, the nitrobenzene is reduced, in which the 2 hydrogen atom replaces the oxygen atom from the nitro group and forms the nitroso benzene.
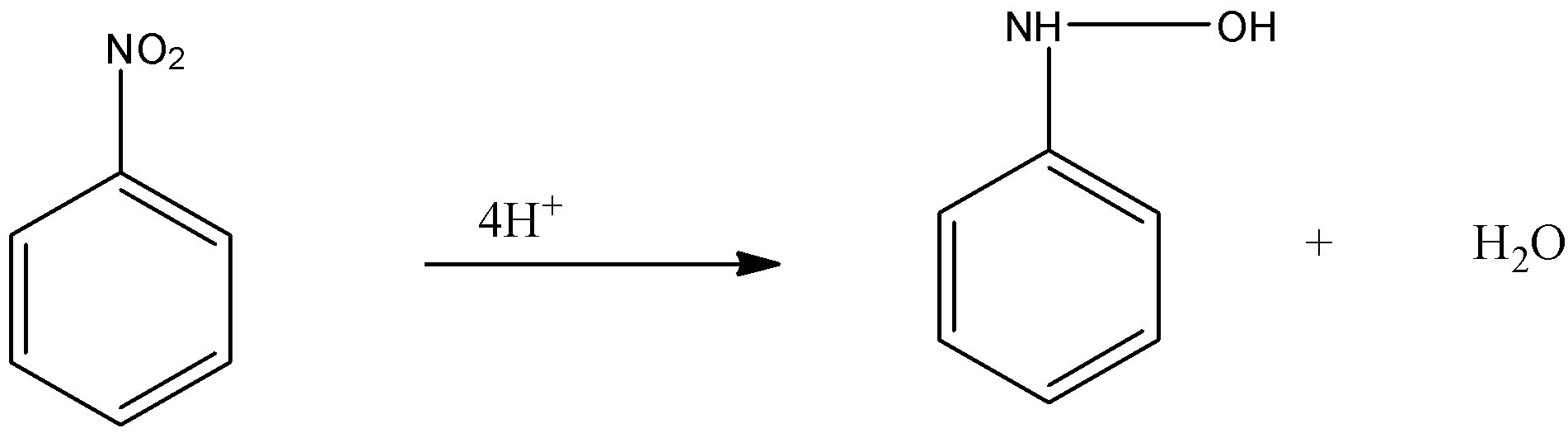
-Now, this nitroso benzene is again reduced and the oxygen atom present with the nitrogen group is replaced by the 4 hydrogen and forms N- phenylhydroxylamine.
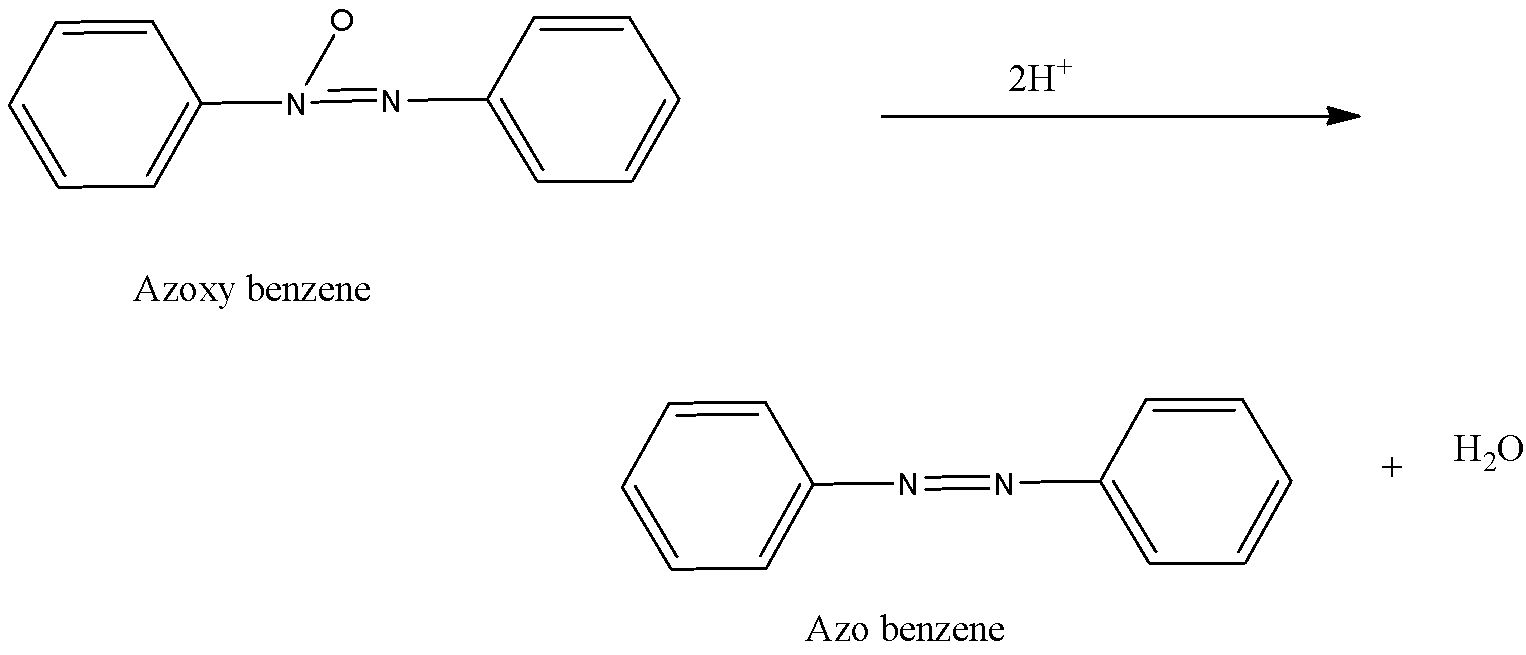
-Now, the N- phenylhydroxylamine and nitroso benzene combine to form azoxybenzene after which it undergoes reduction with the help of 2 hydrogen atoms and forms azo benzene i.e.
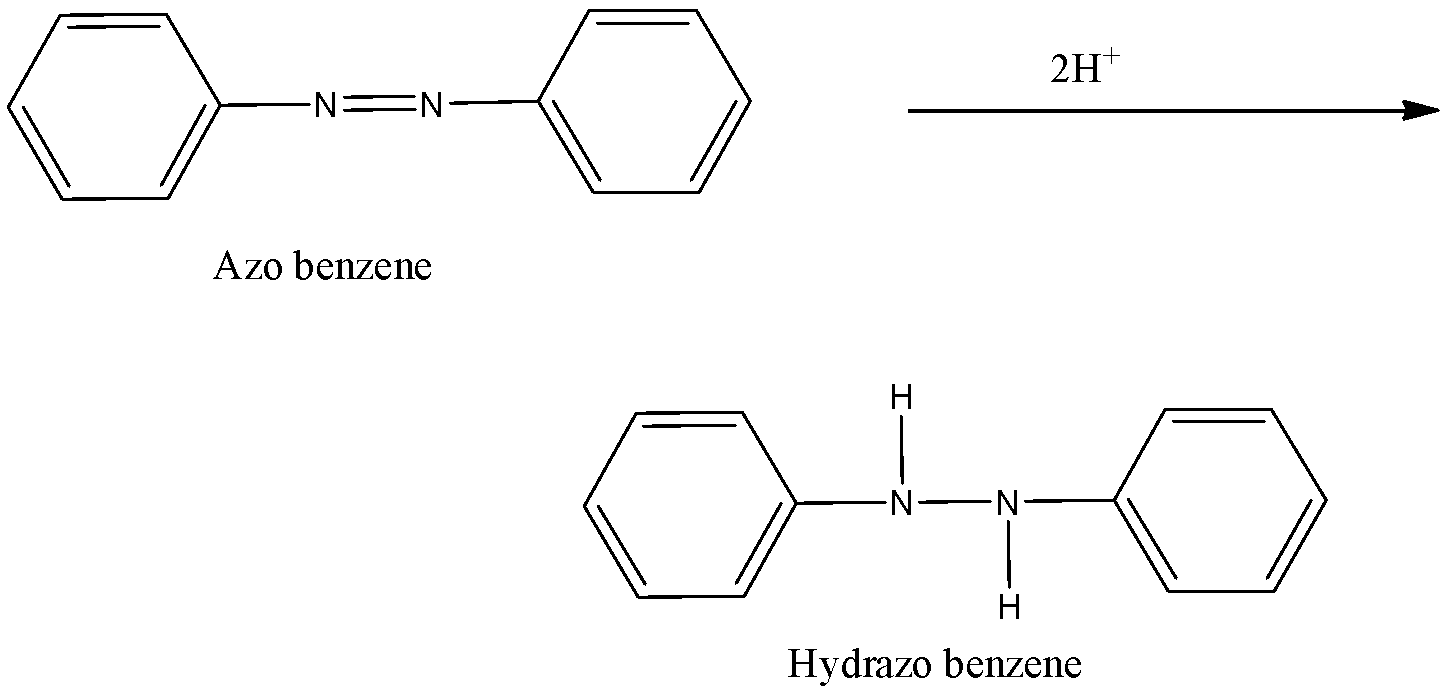
-Now, in the final step, the azobenzene is again by the introduction of two hydrogen atoms on the nitrogen. The double bond present between nitrogen atoms break and the attachment of hydrogen atom take place and the structure is known as hydrazo benzene.
-So, from the above process, we can calculate the no. of moles of hydrogen atoms that were responsible for the reduction process that is 10 (2 + 4 + 2 + 2).
So, the correct answer is “Option A”.
Note: Nitrobenzene can also be used in the synthesis of aniline. Aniline is the 6 membered ring structure with an amine group attached to it and has a molecular formula of ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{H}}_{2}}$. Azobenzene can also be produced with the help of $\text{LiAl}{{\text{H}}_{4}}$ that is a strong reducing agent.
Complete Solution:
-The process of formation of hydrazobenzene includes a total of five steps in which the reduction of every compound takes place.
-Firstly, the nitrobenzene is reduced, in which the 2 hydrogen atom replaces the oxygen atom from the nitro group and forms the nitroso benzene.

-Now, this nitroso benzene is again reduced and the oxygen atom present with the nitrogen group is replaced by the 4 hydrogen and forms N- phenylhydroxylamine.

-Now, the N- phenylhydroxylamine and nitroso benzene combine to form azoxybenzene after which it undergoes reduction with the help of 2 hydrogen atoms and forms azo benzene i.e.

-Now, in the final step, the azobenzene is again by the introduction of two hydrogen atoms on the nitrogen. The double bond present between nitrogen atoms break and the attachment of hydrogen atom take place and the structure is known as hydrazo benzene.

-So, from the above process, we can calculate the no. of moles of hydrogen atoms that were responsible for the reduction process that is 10 (2 + 4 + 2 + 2).
So, the correct answer is “Option A”.
Note: Nitrobenzene can also be used in the synthesis of aniline. Aniline is the 6 membered ring structure with an amine group attached to it and has a molecular formula of ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{H}}_{2}}$. Azobenzene can also be produced with the help of $\text{LiAl}{{\text{H}}_{4}}$ that is a strong reducing agent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




