
What is the number of isomers for disubstituted borazine $B_3N_3H_4X_2$.
Answer
504.6k+ views
Hint: Isomers in chemistry are molecules or polyatomic ions that have the same molecular formula — that is, the same number of atoms of each element — but different atomic configurations in space. Isomerism refers to the existence or potential of isomers. Isomers don't always have the same chemical or physical characteristics as one another. Structural or constitutional isomerism, in which the bonds between the atoms differ, and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative locations of the atoms differ, are the two primary types of isomerism.
Complete answer:
Borazine (sometimes spelled borazole) is a polar inorganic molecule having the formula $B_3N_3H_4X_2$. The three BH units and three NH units alternate in this cyclic molecule. With benzene, the chemical is isoelectronic and structurally identical. Borazine is sometimes referred to as "inorganic benzene" because of this. Borazine, like benzene, is a colourless liquid with an aromatic odour.
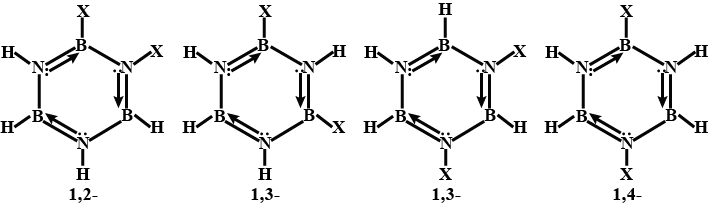
Borazine is frequently referred to as "inorganic benzene" since it is isoelectronic with benzene and has comparable connections. Because of the electronegativity mismatch between boron and nitrogen, this comparison is not strictly valid. The bond lengths within the borazine ring are all equal at 1.429, a characteristic shared by benzene, according to X-ray crystallographic structural findings. The borazine ring, on the other hand, does not form a complete hexagon. The boron atoms have a bond angle of $117.1^o$, whereas the nitrogen atoms have a bond angle of $122.9^o$, giving the molecule unique symmetry. There are four potential isomers, as indicated in the diagram.
Because there are four reactive hydrogen ions in dispersed borazine, there are four potential isomers.
Note:
The NBO analysis of borazine indicates that it has a low aromaticity. The B-N bonds in the ring are somewhat displaced from the nuclear axes in the NBO model, and B and N have significant charge differences. Natural chemical shielding (NCS) study adds to the evidence for aromaticity by showing that the B-N bond contributes to magnetic shielding. Based on NBO orbitals, calculations reveal that this connection allows for a weak ring current that partially counteracts a magnetic field simulated at the core of the borazine ring. Delocalization is suggested by a tiny ring current.
Complete answer:
Borazine (sometimes spelled borazole) is a polar inorganic molecule having the formula $B_3N_3H_4X_2$. The three BH units and three NH units alternate in this cyclic molecule. With benzene, the chemical is isoelectronic and structurally identical. Borazine is sometimes referred to as "inorganic benzene" because of this. Borazine, like benzene, is a colourless liquid with an aromatic odour.
Borazine is frequently referred to as "inorganic benzene" since it is isoelectronic with benzene and has comparable connections. Because of the electronegativity mismatch between boron and nitrogen, this comparison is not strictly valid. The bond lengths within the borazine ring are all equal at 1.429, a characteristic shared by benzene, according to X-ray crystallographic structural findings. The borazine ring, on the other hand, does not form a complete hexagon. The boron atoms have a bond angle of $117.1^o$, whereas the nitrogen atoms have a bond angle of $122.9^o$, giving the molecule unique symmetry. There are four potential isomers, as indicated in the diagram.
Because there are four reactive hydrogen ions in dispersed borazine, there are four potential isomers.

Note:
The NBO analysis of borazine indicates that it has a low aromaticity. The B-N bonds in the ring are somewhat displaced from the nuclear axes in the NBO model, and B and N have significant charge differences. Natural chemical shielding (NCS) study adds to the evidence for aromaticity by showing that the B-N bond contributes to magnetic shielding. Based on NBO orbitals, calculations reveal that this connection allows for a weak ring current that partially counteracts a magnetic field simulated at the core of the borazine ring. Delocalization is suggested by a tiny ring current.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




