
Number of geometrical isomers of ${\left[ {Pt\left( {NH_3} \right)\left( {NO_2} \right)Py\left( {NH_2OH} \right)} \right]^+}$ is
A. 2
B. 3
C. 4
D. 5
Answer
574.5k+ views
Hint: In the given question we have to recognize the isomeric structures of the given complex of geometrical form. When a restricted rotation is present somewhere in a molecule it is said to be a geometrical isomerism and the compound is called a geometrical isomer.
Complete step by step solution:
Isomers are those molecules which have the same molecular formula whereas have a different array of the atoms in space. That contains any different arrangements which are simply due to the rotation of molecules as a whole, or rotation of particular bonds.
In the given complex ${\left[ {Pt\left( {NH_3} \right)\left( {NO_2} \right)Py\left( {NH_2OH} \right)} \right]^+}$ Pt is the central atom and is connected with four different ligand or we call groups therefore it will have 3 different structure according to their functional group attached to it. These are known to be their geometrical isomers of the complex.
Therefore, there are 3 geometrical isomers of the given complex.
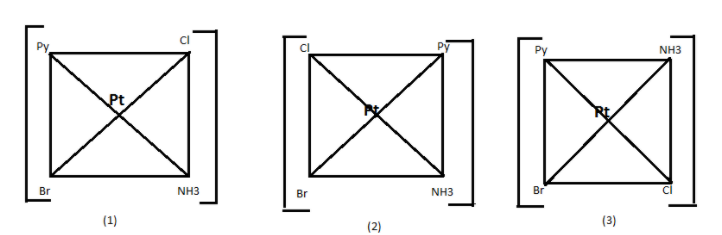
Hence, option (B) is a correct answer.
Note: Geometric isomerism is also known as cis-trans isomerism or E-Z isomerism. The geometrical isomers occur where they have a restricted rotation somewhere in a molecule. To recognize the possibility of a geometric isomerism we obviously need to detect or have a restricted rotation somewhere in the complex of a molecule.
Complete step by step solution:
Isomers are those molecules which have the same molecular formula whereas have a different array of the atoms in space. That contains any different arrangements which are simply due to the rotation of molecules as a whole, or rotation of particular bonds.
In the given complex ${\left[ {Pt\left( {NH_3} \right)\left( {NO_2} \right)Py\left( {NH_2OH} \right)} \right]^+}$ Pt is the central atom and is connected with four different ligand or we call groups therefore it will have 3 different structure according to their functional group attached to it. These are known to be their geometrical isomers of the complex.
Therefore, there are 3 geometrical isomers of the given complex.

Hence, option (B) is a correct answer.
Note: Geometric isomerism is also known as cis-trans isomerism or E-Z isomerism. The geometrical isomers occur where they have a restricted rotation somewhere in a molecule. To recognize the possibility of a geometric isomerism we obviously need to detect or have a restricted rotation somewhere in the complex of a molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




