
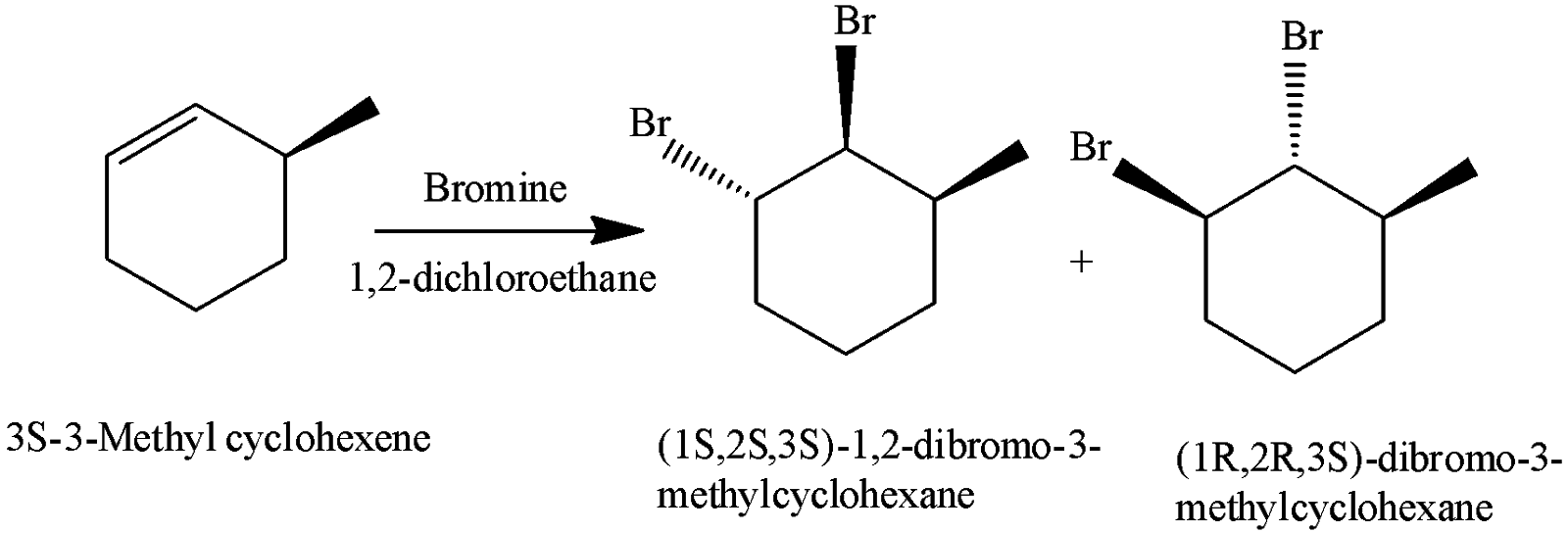
Number of dibromo derivatives products when bromine is added to \[(3S) - 3 - \] methylcyclohexene in $1,2 - $ dichloroethane are:
$
A)2 \\
B)3 \\
C)4 \\
D)6 \\
$
Answer
509.7k+ views
Hint: This is a bromination reaction. It is a reaction in which bromine are introduced into a molecule. It is an oxidation reaction, because each carbon goes from being bound to another carbon to bromine. Bromination of a benzene is a free radical substitution reaction.
Complete answer:
The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as $C{l_2}$ or $B{r_2}$ is added to a molecule after breaking the carbon to carbon double bond. The halides add to neighboring carbons from opposite faces of the molecule.
The number of dibromo derivatives products when bromine is added to \[(3S) - 3 - \]methyl cyclohexene in $1,2 - $dichloroethane are $2$. They are $(1S,2S,3S) - 1,2, - $dibromo$ - 3 - $methyl cyclohexane and $(1R,2R,3S) - $dibromo$ - 3 - $methyl cyclohexane.
$B{r_2}$ is far more soluble than in water. Bromine and $1,2 - $dichloroethane are both nonpolar, with the only forces present being dispersion forces. They are also very comparable and dissolve like they prevail.
So, the correct answer is $B)2$.
Additional information:
One of the significant employments of bromine is a water purifier/sanitizer, as an option in contrast to chlorine. Brominated compounds are utilized for water treatment in pools and hot tubs and are additionally used to control green growth and bacterial development in modern cycles.
Note:
Bromine is destructive to human tissue in a liquid state and its favors aggravate eyes and throat. Bromine fumes are harmful with inward breath. Yet, natural bromines can likewise harm organs like liver, kidneys, lungs and milt and they can cause stomach and gastrointestinal failure.
Complete answer:
The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as $C{l_2}$ or $B{r_2}$ is added to a molecule after breaking the carbon to carbon double bond. The halides add to neighboring carbons from opposite faces of the molecule.
The number of dibromo derivatives products when bromine is added to \[(3S) - 3 - \]methyl cyclohexene in $1,2 - $dichloroethane are $2$. They are $(1S,2S,3S) - 1,2, - $dibromo$ - 3 - $methyl cyclohexane and $(1R,2R,3S) - $dibromo$ - 3 - $methyl cyclohexane.

$B{r_2}$ is far more soluble than in water. Bromine and $1,2 - $dichloroethane are both nonpolar, with the only forces present being dispersion forces. They are also very comparable and dissolve like they prevail.
So, the correct answer is $B)2$.
Additional information:
One of the significant employments of bromine is a water purifier/sanitizer, as an option in contrast to chlorine. Brominated compounds are utilized for water treatment in pools and hot tubs and are additionally used to control green growth and bacterial development in modern cycles.
Note:
Bromine is destructive to human tissue in a liquid state and its favors aggravate eyes and throat. Bromine fumes are harmful with inward breath. Yet, natural bromines can likewise harm organs like liver, kidneys, lungs and milt and they can cause stomach and gastrointestinal failure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




