
Number of compounds which can give Haloform reaction:
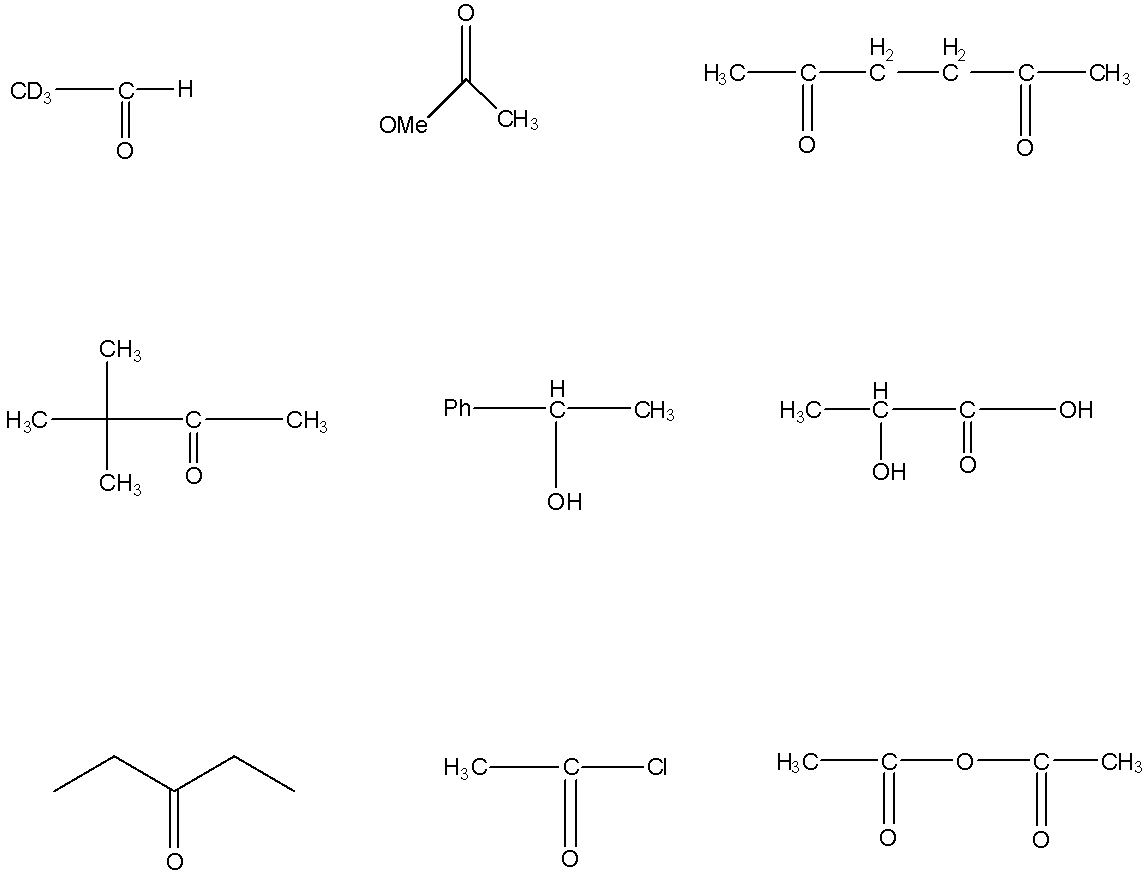

Answer
577.8k+ views
Hint: Haloform reaction is a chemical reaction that is used to detect the presence of $-COC{{H}_{3}}$ group in the molecule. If the $-CO$ group that is connected to the methyl group is connected to another oxygen atom then the compound will not give the haloform reaction.
Complete answer:
Haloform reaction is a chemical reaction in which the haloform like chloroform, bromoform, or iodoform is produced when the organic compound is treated with halogens like chlorine, bromine, or iodine and a base. The compounds that have $-COC{{H}_{3}}$ group in the molecule, only those compounds will give the haloform reaction. If the $-CO$ group that is connected to the methyl group is connected to another oxygen atom then the compound will not give the haloform reaction. In a haloform reaction, there is a formation of precipitate that indicates the presence of $-COC{{H}_{3}}$ group.
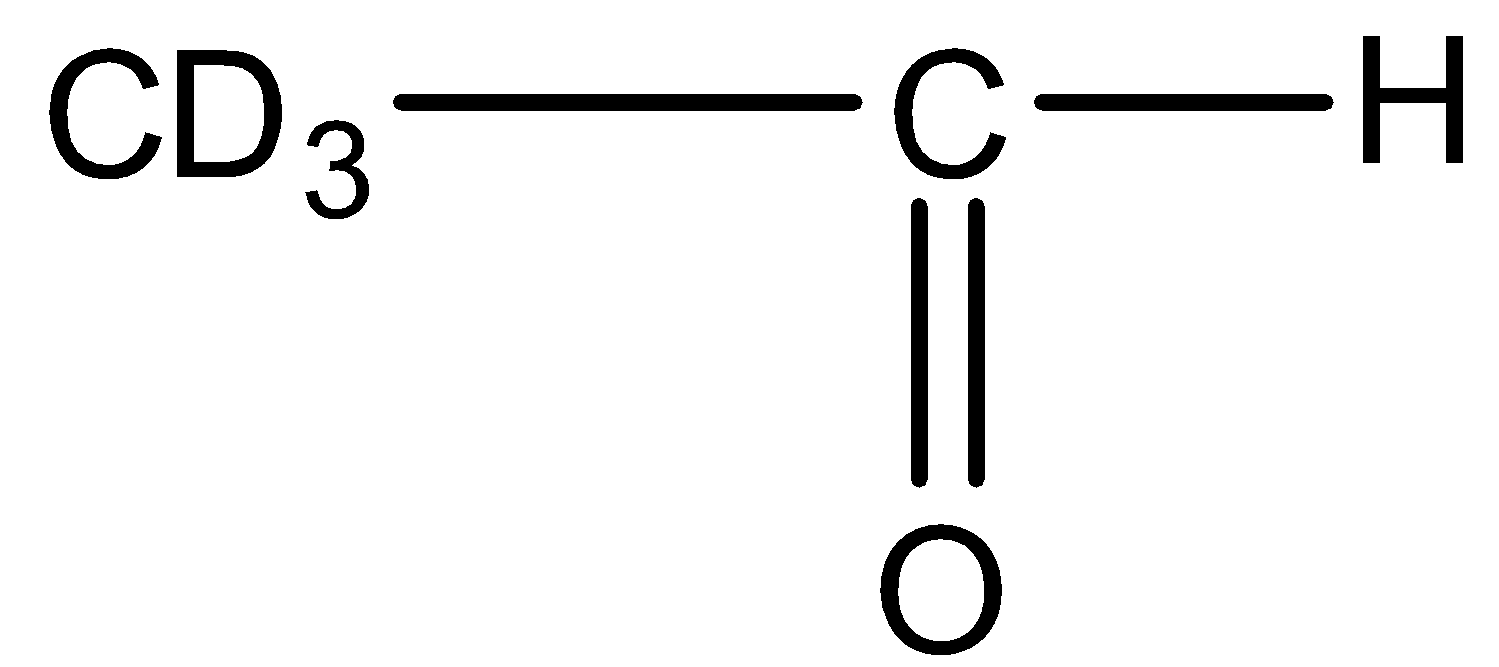
This will give the haloform reaction because there is a $-COC{{D}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom. D is the isotope of hydrogen so it will give the haloform reaction.
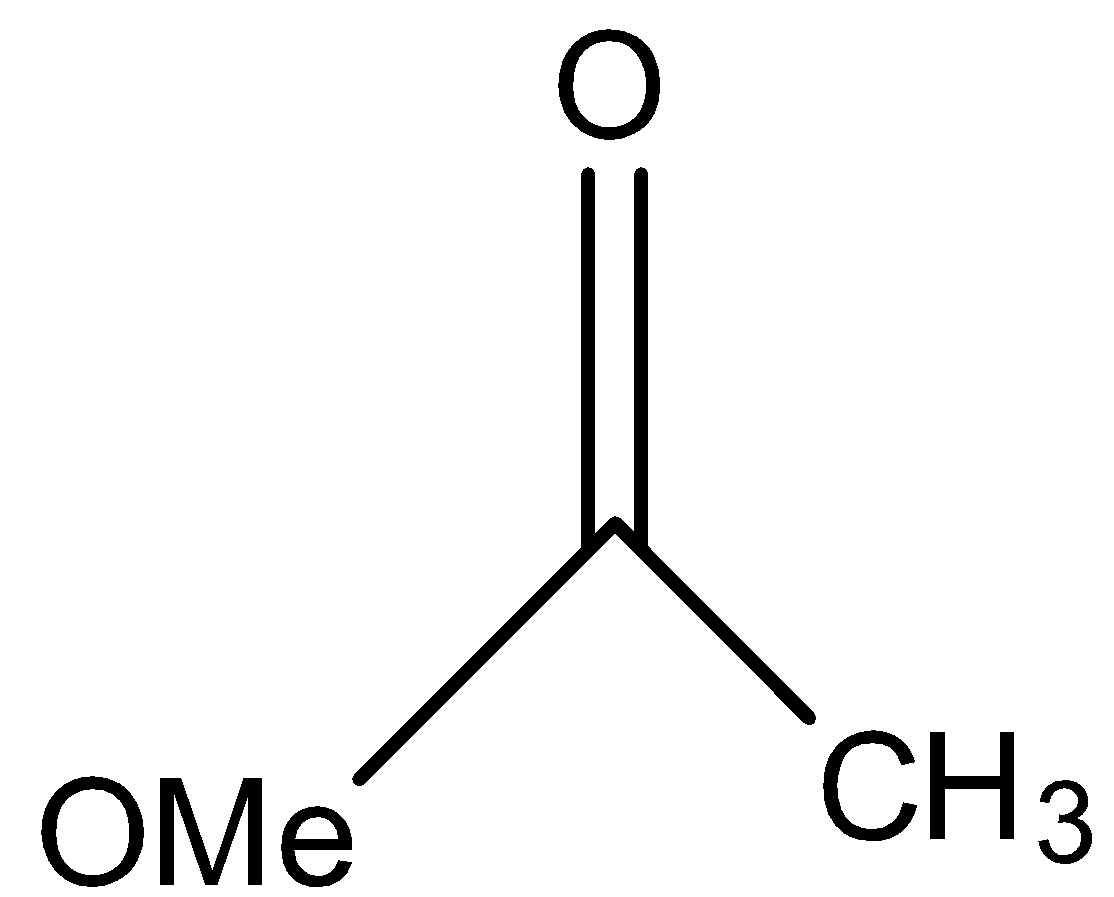
This compound will not give the haloform reaction because the $-COC{{H}_{3}}$ group is attached to another oxygen atom.
This will give the haloform reaction because there is $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.
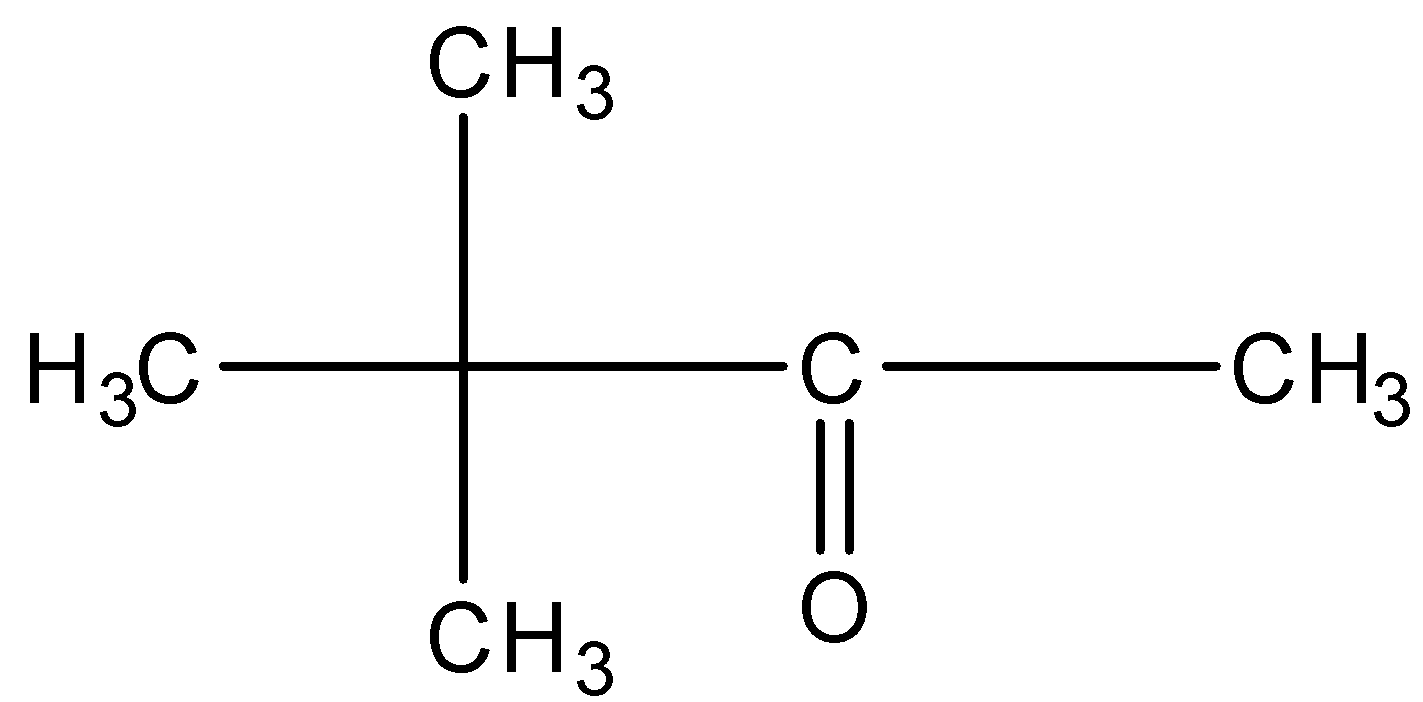
This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.
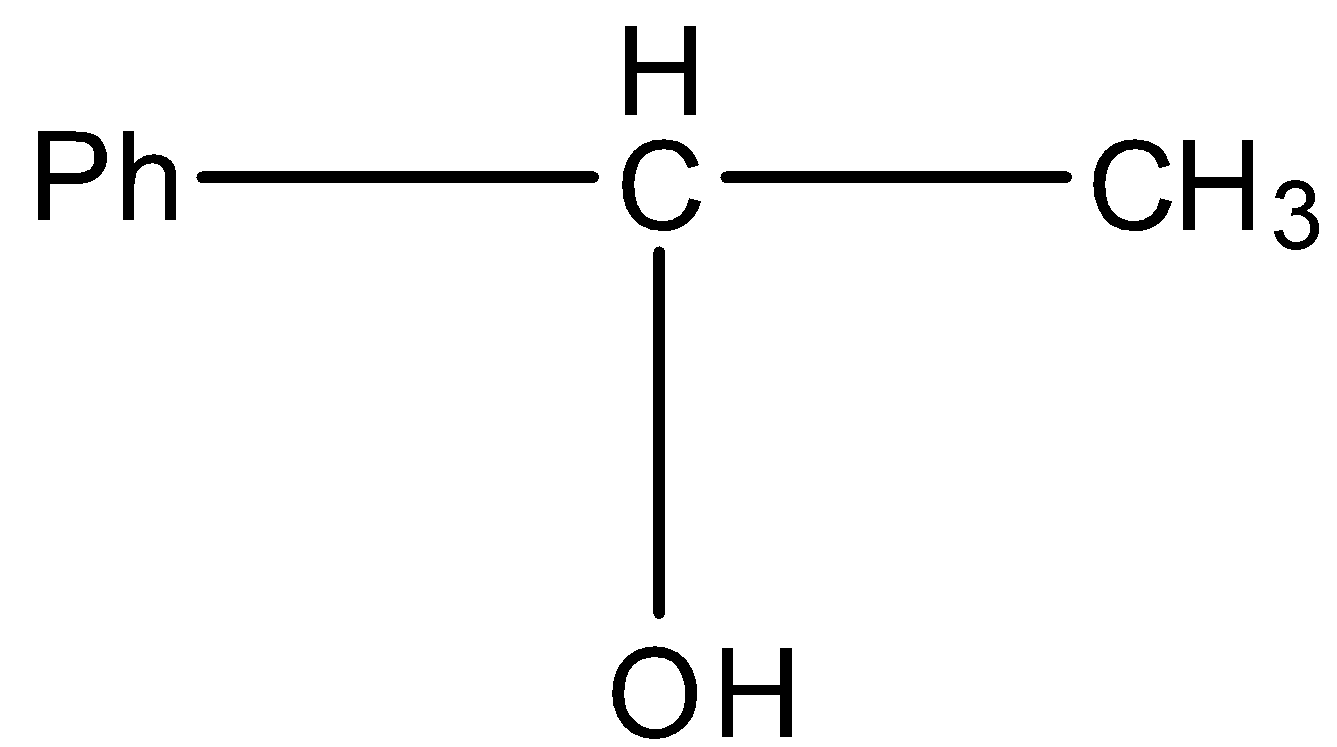
This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

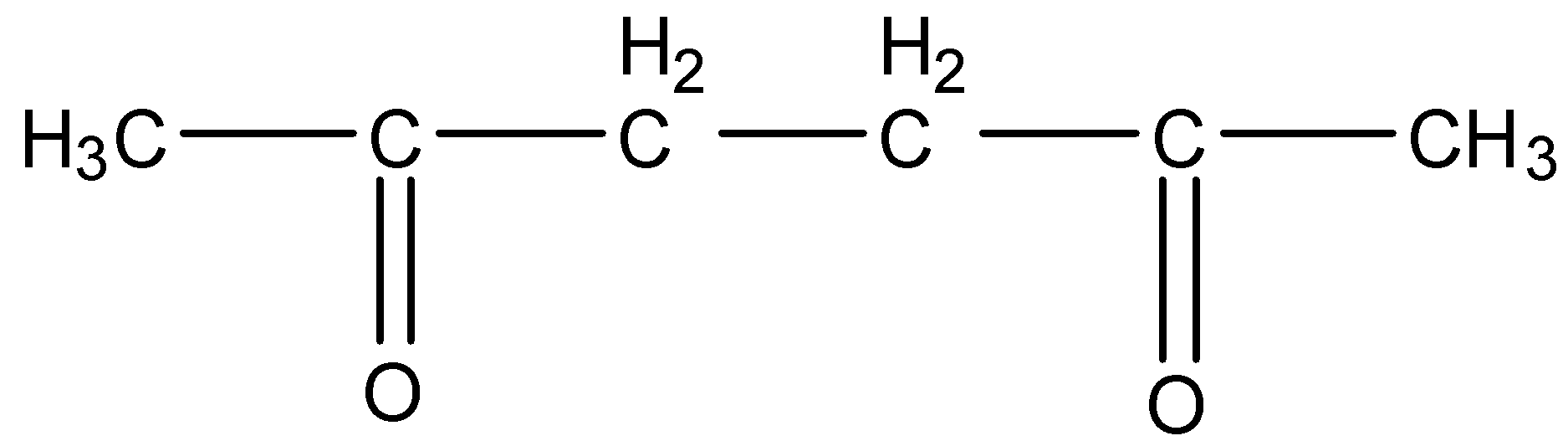
This compound will not give a haloform reaction because it doesn’t have the $-COC{{H}_{3}}$ group.
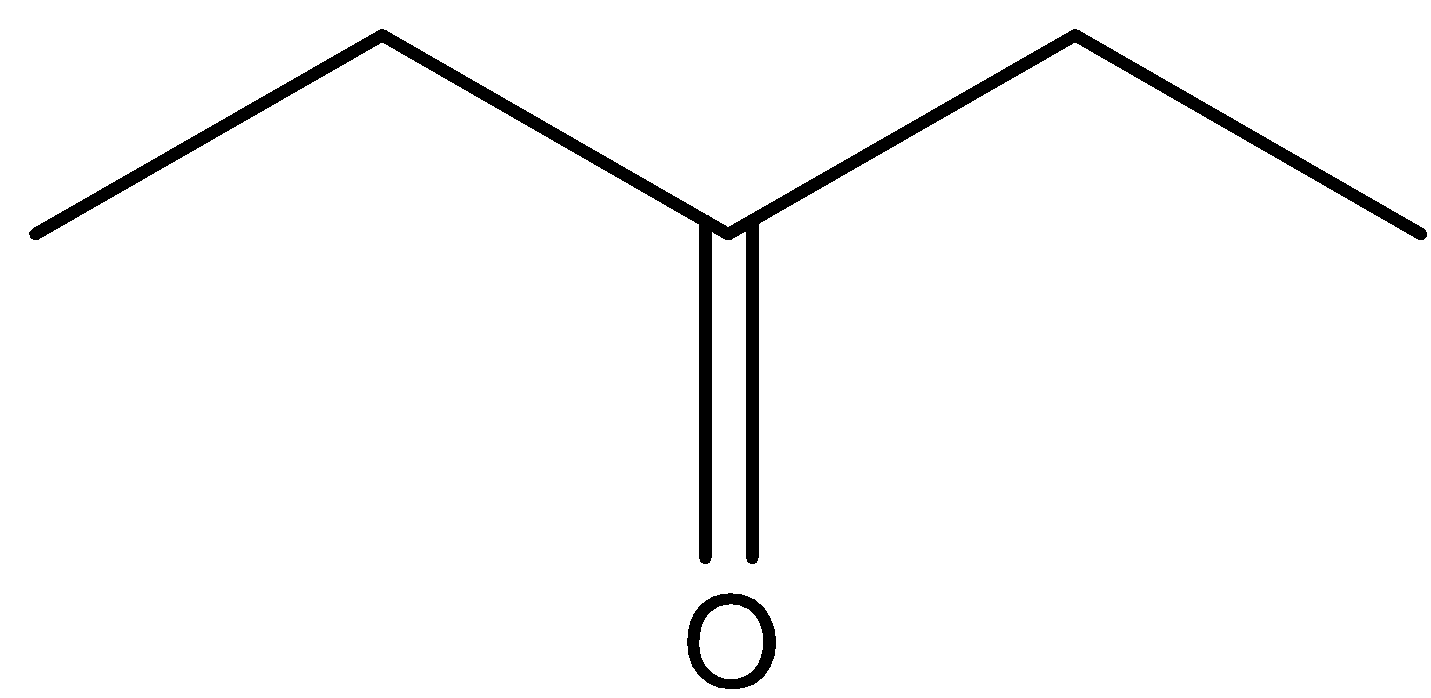
This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.
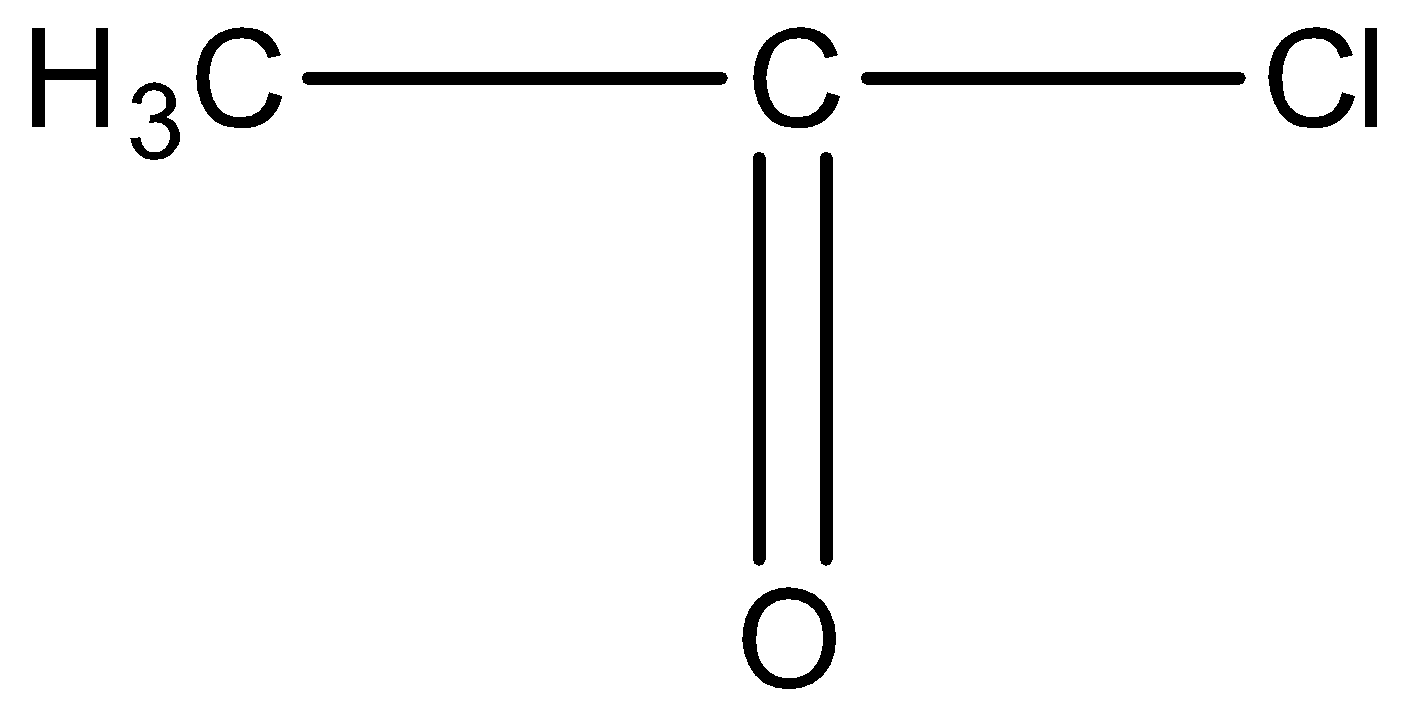
This compound will not give the haloform reaction because the $-COC{{H}_{3}}$ group is attached to another oxygen atom.
So six compounds can give a haloform reaction.
Note:
In the laboratory, this reaction is very helpful in finding the presence of methyl ketone or secondary alcohol in the molecule and industrially this reaction is very helpful in making iodoform, bromoform, and chloroform.
Complete answer:
Haloform reaction is a chemical reaction in which the haloform like chloroform, bromoform, or iodoform is produced when the organic compound is treated with halogens like chlorine, bromine, or iodine and a base. The compounds that have $-COC{{H}_{3}}$ group in the molecule, only those compounds will give the haloform reaction. If the $-CO$ group that is connected to the methyl group is connected to another oxygen atom then the compound will not give the haloform reaction. In a haloform reaction, there is a formation of precipitate that indicates the presence of $-COC{{H}_{3}}$ group.

This will give the haloform reaction because there is a $-COC{{D}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom. D is the isotope of hydrogen so it will give the haloform reaction.

This compound will not give the haloform reaction because the $-COC{{H}_{3}}$ group is attached to another oxygen atom.

This will give the haloform reaction because there is $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

This compound will not give a haloform reaction because it doesn’t have the $-COC{{H}_{3}}$ group.

This will give the haloform reaction because there is a $-COC{{H}_{3}}$ group and the $-CO$ group is not attached to any other oxygen atom.

This compound will not give the haloform reaction because the $-COC{{H}_{3}}$ group is attached to another oxygen atom.
So six compounds can give a haloform reaction.
Note:
In the laboratory, this reaction is very helpful in finding the presence of methyl ketone or secondary alcohol in the molecule and industrially this reaction is very helpful in making iodoform, bromoform, and chloroform.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




