
When nitrobenzene is reduced with a metal and acid (\[Sn/HCl\],\[Zn/HCl\] etc.), which of the following product(s) is obtained?
A. aniline
B. nitrosobenzene
C. phenylhydroxylamine
D. all the above
Answer
578.1k+ views
Hint: Reduction is referred to as the addition of hydrogen. The metals such as tin and zinc are highly reducing in nature and are used as reducing agents.
Complete step by step answer:
When nitrobenzene is treated with a metal in presence of \[HCl\] under heating conditions, addition of hydrogen takes place. The product formed is aniline by replacing the oxygen atoms.
The reaction occurs in two steps. At first the phenyl ammonium ion is formed by picking protons from the acid used in the reaction. The corresponding half reactions are as follows:
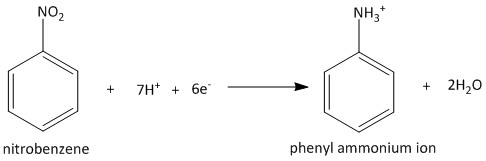
The electrons come from the metal ions which undergo oxidation during the reaction.
\[Sn \to S{n^{2 + }} + 2{e^ - }\]
\[S{n^{2 + }} \to S{n^{4 + }} + 2{e^ - }\]
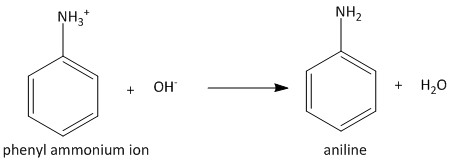
The phenyl ammonium ion produced from nitrobenzene is converted to phenylamine or aniline on basification or basic workup. The corresponding reaction is as follows:
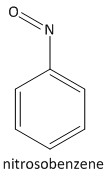
Nitrosobenzene is an intermediate which is formed during the reduction reaction. But it is impossible to isolate it as in pure form due to unstable nature. It rather transforms into aniline in the reaction mixture. The structure of the intermediate is:
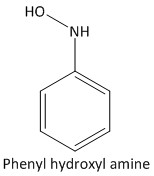
Phenyl hydroxylamine is also an intermediate which is formed by transfer of two protons to nitrobenzene. It is also an unstable intermediate and transforms into aniline during the reaction. The structure of the intermediate is:
Thus the product is aniline when nitrobenzene is reduced with a metal and acid (\[Sn/HCl\],\[Zn/HCl\] etc.).
Note:
Such reduction is also achieved using hydrogenation which is more favored than these metal-acid mixture based reactions. The isolation of the product is easier. The filtration of the reaction mixture and evaporation of the filtrate yields the reduced product. The workup of metal-acid mixture is very tedious.
Complete step by step answer:
When nitrobenzene is treated with a metal in presence of \[HCl\] under heating conditions, addition of hydrogen takes place. The product formed is aniline by replacing the oxygen atoms.
The reaction occurs in two steps. At first the phenyl ammonium ion is formed by picking protons from the acid used in the reaction. The corresponding half reactions are as follows:

The electrons come from the metal ions which undergo oxidation during the reaction.
\[Sn \to S{n^{2 + }} + 2{e^ - }\]
\[S{n^{2 + }} \to S{n^{4 + }} + 2{e^ - }\]
The phenyl ammonium ion produced from nitrobenzene is converted to phenylamine or aniline on basification or basic workup. The corresponding reaction is as follows:

Nitrosobenzene is an intermediate which is formed during the reduction reaction. But it is impossible to isolate it as in pure form due to unstable nature. It rather transforms into aniline in the reaction mixture. The structure of the intermediate is:

Phenyl hydroxylamine is also an intermediate which is formed by transfer of two protons to nitrobenzene. It is also an unstable intermediate and transforms into aniline during the reaction. The structure of the intermediate is:

Thus the product is aniline when nitrobenzene is reduced with a metal and acid (\[Sn/HCl\],\[Zn/HCl\] etc.).
Note:
Such reduction is also achieved using hydrogenation which is more favored than these metal-acid mixture based reactions. The isolation of the product is easier. The filtration of the reaction mixture and evaporation of the filtrate yields the reduced product. The workup of metal-acid mixture is very tedious.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




