
Nitration of which among the following compounds yield cyclonite?
(A) Formaldehyde
(B) Benzaldehyde
(C) Urotropine
(D) Acetaldehyde-ammonia
Answer
584.7k+ views
Hint: Cyclonite is also known as RDX and has a molecular formula of ${\left( {C{H_2}{N_2}{O_2}} \right)_3}$. It is formed by the nitration of a heterocyclic organic compound which is used in the treatment of urinary tract infection and can be used as a solid fuel. This compound is formed by the reaction of ammonia with formaldehyde.
Complete step by step solution:
-First, we will see what cyclonite is.
Cyclonite is basically an organic substance with a molecular formula of ${\left( {C{H_2}{N_2}{O_2}} \right)_3}$ and is also known as Royal demolition explosive (RDX) or hexogen. Its IUPAC name is 1, 3, 5-trinitro-1, 3, 5-triazinane. Since it is very similar to HMX, it has been chemically classified as nitramide. Its structure is shown below:
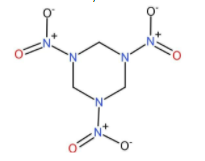
It is highly explosive in nature and thus is used as an explosive alone as well as in combination with other explosives. It is also used in phlegmatizers as desensitizers to reduce the sensitivity of the explosives. It is highly energetic and has high brisance for use in military high explosives.
-Now let us talk about nitration.
Nitration is basically a process in which nitro group is added to an organic compound with the help of a mixture of nitric acid (conc. $HN{O_3}$) and sulphuric acid (conc. ${H_2}S{O_4}$). This mixture leads to the formation of a nitronium ion ($NO_2^ + $) which acts as the active species in this reaction and attacks on the compound which needs to be nitrated.
-We will now talk about the formation of cyclonite. Since we have seen that cyclonite is a 6 membered ring structure, it should have been formed by the nitration of a similar type of molecule. Among the options, there is only one such structure and that is Urotropine.
Urotropine is an organic compound of molecular formula ${\left( {C{H_2}} \right)_6}{N_4}$ and it is itself formed by the reaction of ammonia and formaldehyde. This formation reaction or Urotropine is:
$6HCHO + 4N{H_3} \to {\left( {C{H_2}} \right)_6}{N_4} + 6{H_2}O$
Its structure is:
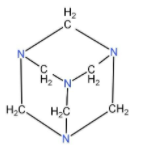
The nitration of Urotropine leads to the formation of cyclonite. This is shown in the following reaction:
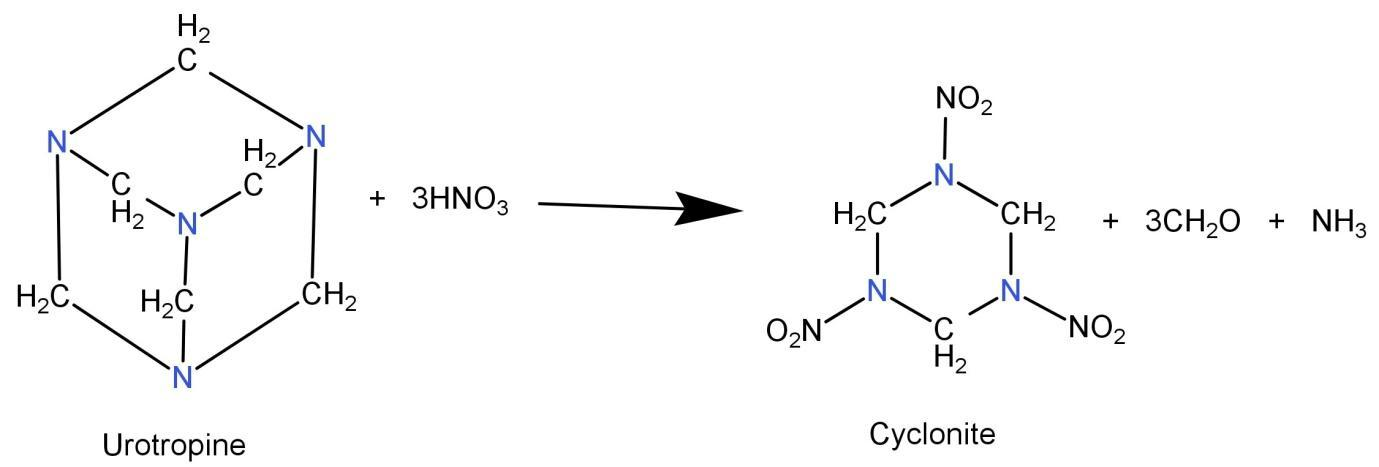
Hence the correct option will be: (C) Urotropine
Note: RDX was used during the World War ll along with TNT to form explosive mixtures like Composition B, Torpex, H6, Cyclotols, etc. It has a large explosive potential for the formation of ${N_2}$ and $C{O_2}$ due to its high nitrogen content and high O: C ratio. It is very explosive and even toxic. It can be degraded by the organisms in sewage sludge and by a fungus named Phanerochaete chrysosporium.
Complete step by step solution:
-First, we will see what cyclonite is.
Cyclonite is basically an organic substance with a molecular formula of ${\left( {C{H_2}{N_2}{O_2}} \right)_3}$ and is also known as Royal demolition explosive (RDX) or hexogen. Its IUPAC name is 1, 3, 5-trinitro-1, 3, 5-triazinane. Since it is very similar to HMX, it has been chemically classified as nitramide. Its structure is shown below:

It is highly explosive in nature and thus is used as an explosive alone as well as in combination with other explosives. It is also used in phlegmatizers as desensitizers to reduce the sensitivity of the explosives. It is highly energetic and has high brisance for use in military high explosives.
-Now let us talk about nitration.
Nitration is basically a process in which nitro group is added to an organic compound with the help of a mixture of nitric acid (conc. $HN{O_3}$) and sulphuric acid (conc. ${H_2}S{O_4}$). This mixture leads to the formation of a nitronium ion ($NO_2^ + $) which acts as the active species in this reaction and attacks on the compound which needs to be nitrated.
-We will now talk about the formation of cyclonite. Since we have seen that cyclonite is a 6 membered ring structure, it should have been formed by the nitration of a similar type of molecule. Among the options, there is only one such structure and that is Urotropine.
Urotropine is an organic compound of molecular formula ${\left( {C{H_2}} \right)_6}{N_4}$ and it is itself formed by the reaction of ammonia and formaldehyde. This formation reaction or Urotropine is:
$6HCHO + 4N{H_3} \to {\left( {C{H_2}} \right)_6}{N_4} + 6{H_2}O$
Its structure is:

The nitration of Urotropine leads to the formation of cyclonite. This is shown in the following reaction:

Hence the correct option will be: (C) Urotropine
Note: RDX was used during the World War ll along with TNT to form explosive mixtures like Composition B, Torpex, H6, Cyclotols, etc. It has a large explosive potential for the formation of ${N_2}$ and $C{O_2}$ due to its high nitrogen content and high O: C ratio. It is very explosive and even toxic. It can be degraded by the organisms in sewage sludge and by a fungus named Phanerochaete chrysosporium.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




