
Nitration of benzoic acid gives:
A. Ortho and para nitrobenzoic acid
B. Meta nitrobenzoic acid
C. Nitrobenzene
D. None.
Answer
613.5k+ views
Hint- In order to solve the given problem, first we will see what is benzoic acid and further we will see the process of nitration for the benzene and also the products formed after the nitration. On the basis of this process, we will select the correct option amongst the given ones.
Complete answer:
Benzoic acid is an aromatic acid that is susceptible to the normal electrophilic benzene ring replacement reactions such as nitration.
The nitration of benzoic acid thus results in the formation of a compound called 3-Nitrobenzoic acid.
That is because the group "-COOH" present in benzoic acid is electron-withdrawal, thus it is meta-directing.
Because of this the electrophilic substitution reaction happens in the benzoic acid meta position.
The chemical equation involved in the reaction is:
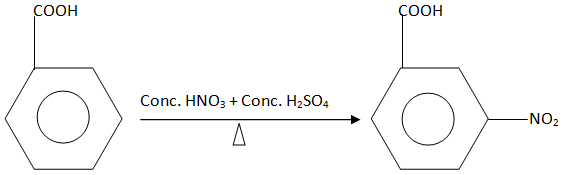
Hence, nitration of benzoic acid gives meta nitrobenzoic acid.
So, the correct option is B.
Additional information-
Aromatic ring substituents affect the extent of this aromatic electrophilic substitution. The deactivation of groups such as certain nitro groups has an effect of electron-removal. These groups deactivate the reaction (slow) and steer the electrophilic nitronium ion to attack the meta aromatic position. The meta-directing substituents are deactivated by sulfonyl, cyano groups, keto, esters, and carboxylates. Nitration can be stimulated by activating groups such as amino, hydroxy and methyl groups, resulting in para and ortho isomers as well as amides and ethers.
Note- Nitration is a general class of chemical processes in which a nitro group is introduced into an organic compound. The term is often wrongly applied to the specific cycle of nitrate esters formation between alcohols and nitric acid (as happens in nitroglycerine synthesis). Benzene is a synthetic chemical and is commonly used. Benzene is present in crude oil and is a major part of petrol. It's used to make plastics, resins, synthetic fibers, rubber lubricants, dyes, detergents, drugs and pesticides.
Complete answer:
Benzoic acid is an aromatic acid that is susceptible to the normal electrophilic benzene ring replacement reactions such as nitration.
The nitration of benzoic acid thus results in the formation of a compound called 3-Nitrobenzoic acid.
That is because the group "-COOH" present in benzoic acid is electron-withdrawal, thus it is meta-directing.
Because of this the electrophilic substitution reaction happens in the benzoic acid meta position.
The chemical equation involved in the reaction is:

Hence, nitration of benzoic acid gives meta nitrobenzoic acid.
So, the correct option is B.
Additional information-
Aromatic ring substituents affect the extent of this aromatic electrophilic substitution. The deactivation of groups such as certain nitro groups has an effect of electron-removal. These groups deactivate the reaction (slow) and steer the electrophilic nitronium ion to attack the meta aromatic position. The meta-directing substituents are deactivated by sulfonyl, cyano groups, keto, esters, and carboxylates. Nitration can be stimulated by activating groups such as amino, hydroxy and methyl groups, resulting in para and ortho isomers as well as amides and ethers.
Note- Nitration is a general class of chemical processes in which a nitro group is introduced into an organic compound. The term is often wrongly applied to the specific cycle of nitrate esters formation between alcohols and nitric acid (as happens in nitroglycerine synthesis). Benzene is a synthetic chemical and is commonly used. Benzene is present in crude oil and is a major part of petrol. It's used to make plastics, resins, synthetic fibers, rubber lubricants, dyes, detergents, drugs and pesticides.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




