
How is nicotine extracted from tobacco leaves and solubilized?
Answer
554.4k+ views
Hint: We know that the process for extracting nicotine from vegetable, that to particularly from tobacco leaves, by adjusting water content of tobacco and by treating tobacco so with ammonia or aqueous sulphuric acid for a shorter time period and with help of extracting the acid treated with a liquid or any non-aqueous solvent in the liquid phase and then by separating nicotine from solvent extract. The trace of solvent is separated from extracted vegetable and likewise together with solvent separated from nicare given.
Complete step-by-step answer:Before starting with the extraction process some properties of nicotine we should know, Nicotine is oily, colorless liquid with boiling point around \[247\text{ }{}^\circ C\] Its structure is given below in diagram;
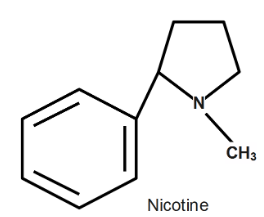
Also it is basic in nature and it already exists in the plant as a form of the salt of various acids.
We can better represent that reaction as:
$Nic+H\to Nic{{H}^{+}}+{{A}^{-}}$
The actual process of extraction of nicotine from tobacco leaves is a simplified version of industrial process given down here:
1.Firstly, cutting down the tobacco leaves to pass a \[6mm\] sieve.
2.After that adding a lime slurry in order to free nicotine from its own salt the reaction is given by :
$Nic{{H}^{+}}+O{{H}^{-}}\to Nic+{{H}_{2}}O$
3.Then we have to heat the slurry with pre superheated pressurized steam.
4.The following process is effectively a steam distillation of the oil then we have to condense the generated vapors.
5.After that extract the nicotine from the condensate form with the help of kerosene.
6.Then extracting the nicotine from kerosene with the adequate amount that is \[25%\] sulfuric acid the reaction is given by:
\[2Nic\left( kerosene \right)~+~{{H}_{2}}S{{O}_{4}}\left( aq \right)\text{ }\to \text{ }{{\left( NicH \right)}_{2}}S{{O}_{4}}\left( aq \right)\]
7.A last using the alkali to adjust the solution to the pH level of six for safe transport inside the metal container.
Note: Note that in the process of extracting the nicotine from material which contains the same which also comprise of treating material with non-aqueous solvent for subsequent nicotine meanwhile separating the nicotine from solvent to extract step which comprise of treating and extraction simultaneously with help of sulfuric acid which is aqueous solution and nicotine sulfate.
Complete step-by-step answer:Before starting with the extraction process some properties of nicotine we should know, Nicotine is oily, colorless liquid with boiling point around \[247\text{ }{}^\circ C\] Its structure is given below in diagram;

Also it is basic in nature and it already exists in the plant as a form of the salt of various acids.
We can better represent that reaction as:
$Nic+H\to Nic{{H}^{+}}+{{A}^{-}}$
The actual process of extraction of nicotine from tobacco leaves is a simplified version of industrial process given down here:
1.Firstly, cutting down the tobacco leaves to pass a \[6mm\] sieve.
2.After that adding a lime slurry in order to free nicotine from its own salt the reaction is given by :
$Nic{{H}^{+}}+O{{H}^{-}}\to Nic+{{H}_{2}}O$
3.Then we have to heat the slurry with pre superheated pressurized steam.
4.The following process is effectively a steam distillation of the oil then we have to condense the generated vapors.
5.After that extract the nicotine from the condensate form with the help of kerosene.
6.Then extracting the nicotine from kerosene with the adequate amount that is \[25%\] sulfuric acid the reaction is given by:
\[2Nic\left( kerosene \right)~+~{{H}_{2}}S{{O}_{4}}\left( aq \right)\text{ }\to \text{ }{{\left( NicH \right)}_{2}}S{{O}_{4}}\left( aq \right)\]
7.A last using the alkali to adjust the solution to the pH level of six for safe transport inside the metal container.
Note: Note that in the process of extracting the nicotine from material which contains the same which also comprise of treating material with non-aqueous solvent for subsequent nicotine meanwhile separating the nicotine from solvent to extract step which comprise of treating and extraction simultaneously with help of sulfuric acid which is aqueous solution and nicotine sulfate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




