
What is the n-factor for the phenol in the following reaction?
$Phenol\xrightarrow{{{(N{{H}_{4}})}_{2}}C{{r}_{2}}{{O}_{7}}}?$
Answer
584.7k+ views
Hint: The n-factor of the compound is counted by the difference in the oxidation number of the compound when it changes from reactant to product. ${{(N{{H}_{4}})}_{2}}C{{r}_{2}}{{O}_{7}}$ is a strong oxidizing agent, hence it oxidizes the phenol molecule.
Complete step by step answer:
Phenol is an aromatic organic compound in which the hydroxyl group or the alcohol group is attached to one of the carbon atoms in the benzene ring. The formula is ${{C}_{6}}{{H}_{5}}OH$. The structure of phenol is given below:
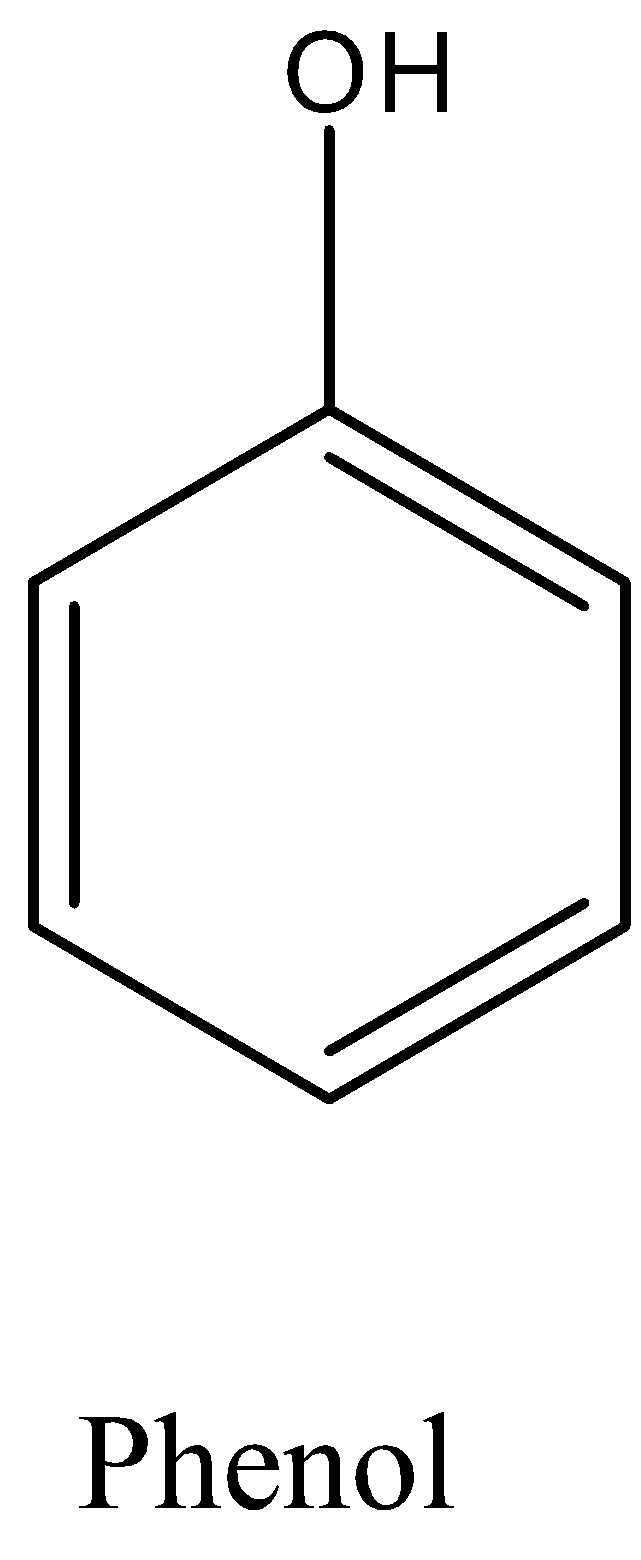
So, in the question phenol is subjected to ammonium dichromate. Ammonium dichromate is a very strong oxidizing agent and easily oxidizes the compound. So, when the phenol is reacted with ammonium dichromate a diketone will be formed. The formed structure is 1,4-Benzoquinone.
The reaction is given below:
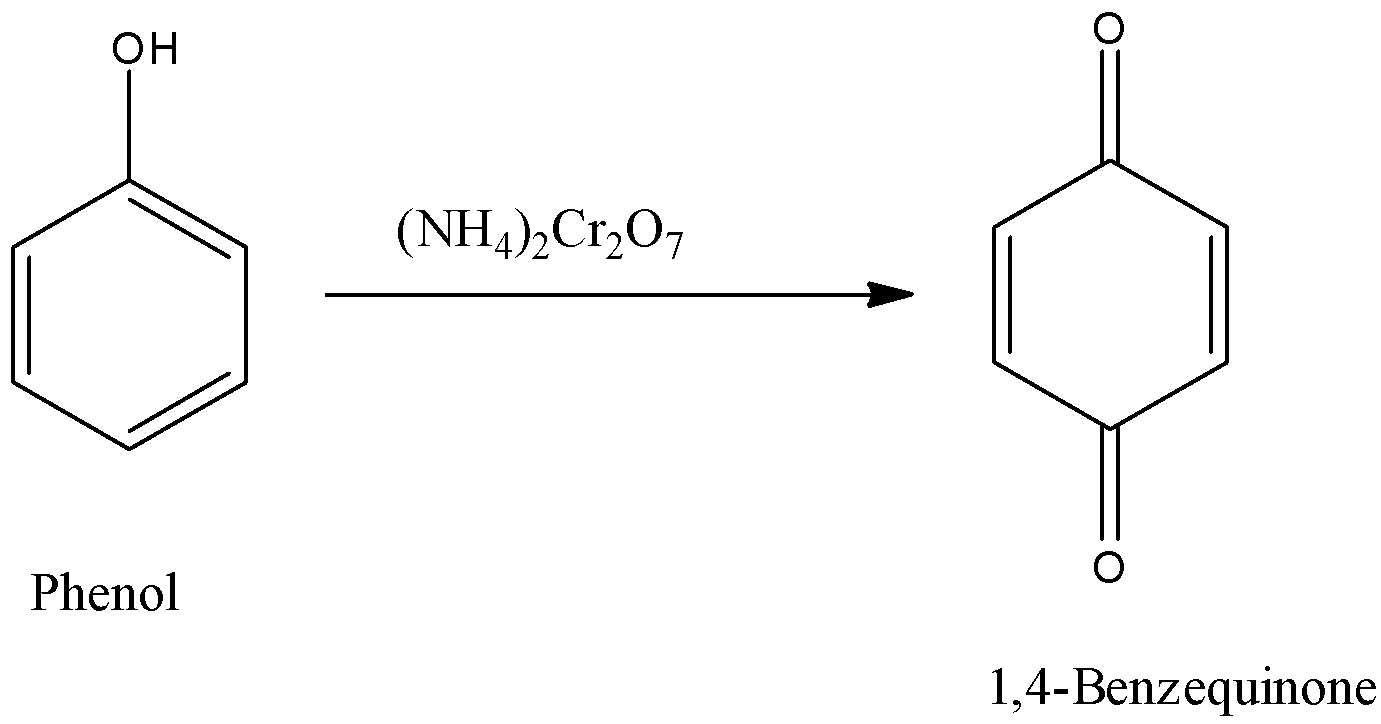
Now we have to calculate the n-factor of phenol.
n-factor can be calculated by observing the change in the oxidation number of the compound, whether oxidation or reduction takes place depending on the reaction. So, in the above reaction, the reactant is phenol, so the oxidation number will be:
$6x+5-2+1=0$
$6x=-4$
And in the above reaction, the product of 1,4-Benzenquinone, so the oxidation number will be:
$6x+4+2(-2)=0$
$6x+4-4=0$
$6x=0$
So, the oxidation state changes from -4 to 0. The difference is 4 between them. Therefore the n-factor of the phenol in the reaction is 4.
Note: Always remember that the n-factor of a compound is always calculated for 1 mole of the compound. If there are n moles in the compound then the number of moles is multiplied to the n-factor.
Complete step by step answer:
Phenol is an aromatic organic compound in which the hydroxyl group or the alcohol group is attached to one of the carbon atoms in the benzene ring. The formula is ${{C}_{6}}{{H}_{5}}OH$. The structure of phenol is given below:

So, in the question phenol is subjected to ammonium dichromate. Ammonium dichromate is a very strong oxidizing agent and easily oxidizes the compound. So, when the phenol is reacted with ammonium dichromate a diketone will be formed. The formed structure is 1,4-Benzoquinone.
The reaction is given below:

Now we have to calculate the n-factor of phenol.
n-factor can be calculated by observing the change in the oxidation number of the compound, whether oxidation or reduction takes place depending on the reaction. So, in the above reaction, the reactant is phenol, so the oxidation number will be:
$6x+5-2+1=0$
$6x=-4$
And in the above reaction, the product of 1,4-Benzenquinone, so the oxidation number will be:
$6x+4+2(-2)=0$
$6x+4-4=0$
$6x=0$
So, the oxidation state changes from -4 to 0. The difference is 4 between them. Therefore the n-factor of the phenol in the reaction is 4.
Note: Always remember that the n-factor of a compound is always calculated for 1 mole of the compound. If there are n moles in the compound then the number of moles is multiplied to the n-factor.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




