
How do you name unsymmetrical anhydrides?
Answer
558.6k+ views
Hint:An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being Acidic in nature.
Complete answer:
We have named unsymmetrical acid anhydrides by naming each component carboxylic acid alphabetically without the word acid, followed by spaces and then the word anhydride.
In simple words we know that the simple acid anhydrides, the two alkyl groups are the same, while in mixed acid anhydrides, the groups R and R' are different.
Example, \[C{{H}_{3}}COOCOC{{H}_{3}}\]
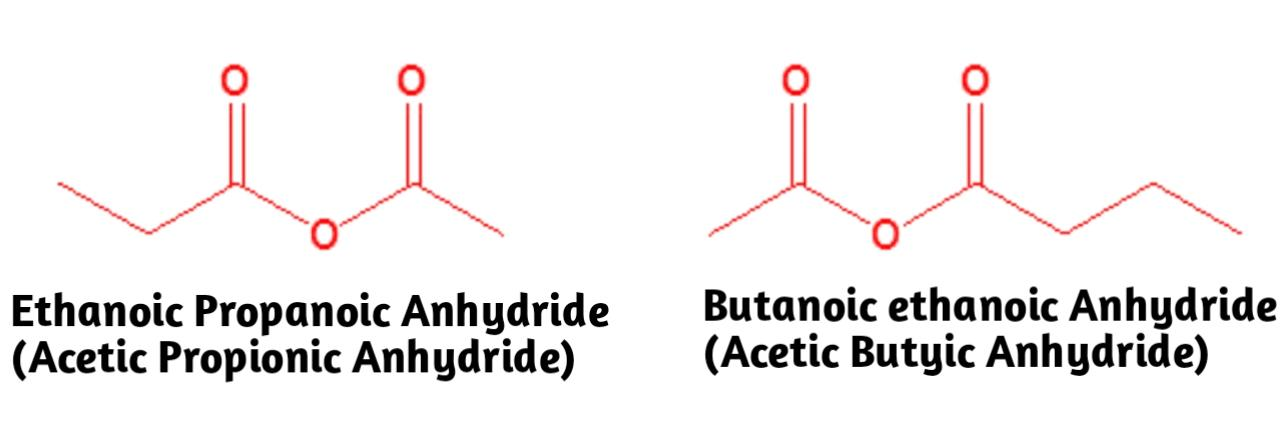
Additional Information:We should know what unsymmetrical anhydrides are: The acid anhydride functional group results when two carboxylic acids combine and lose water anhydride without water. Symmetrical acid anhydrides are named like carboxylic acids except the ending acid is replaced with anhydride. Thus the statement is true for both the IUPAC and Common nomenclature of compounds.
Note:Usually the common mistakes are done in Identification of Acidic and Basic Anhydride.We have to firstly classify that the Anhydrides are functional groups which are basically the derivatives of either acids or bases. Acid anhydrides will have R-COO-CO-R' pattern where R and R' are alkyl groups. While basic anhydrides will not have such a pattern of atomic or molecular connections.
Complete answer:
We have named unsymmetrical acid anhydrides by naming each component carboxylic acid alphabetically without the word acid, followed by spaces and then the word anhydride.
In simple words we know that the simple acid anhydrides, the two alkyl groups are the same, while in mixed acid anhydrides, the groups R and R' are different.
Example, \[C{{H}_{3}}COOCOC{{H}_{3}}\]

Additional Information:We should know what unsymmetrical anhydrides are: The acid anhydride functional group results when two carboxylic acids combine and lose water anhydride without water. Symmetrical acid anhydrides are named like carboxylic acids except the ending acid is replaced with anhydride. Thus the statement is true for both the IUPAC and Common nomenclature of compounds.
Note:Usually the common mistakes are done in Identification of Acidic and Basic Anhydride.We have to firstly classify that the Anhydrides are functional groups which are basically the derivatives of either acids or bases. Acid anhydrides will have R-COO-CO-R' pattern where R and R' are alkyl groups. While basic anhydrides will not have such a pattern of atomic or molecular connections.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




