
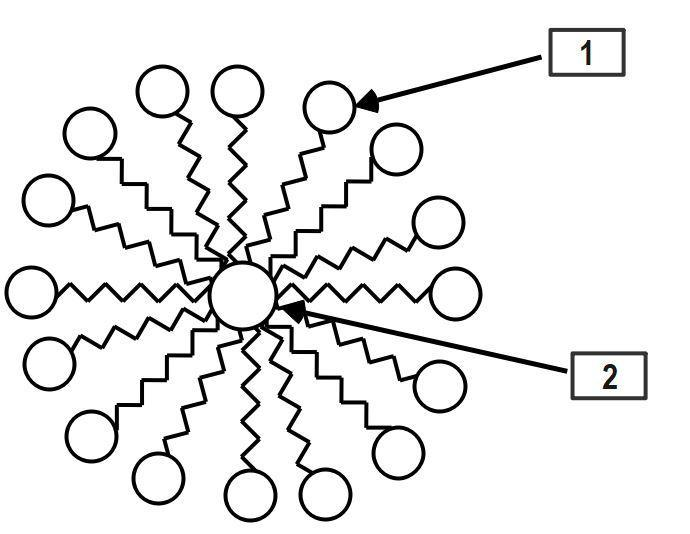
Name the structure shown in the figure. Also label $1$ and \[2.\]

Answer
514.2k+ views
Hint :We know that a colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium. The essential difference between a solution and a colloid is that of particle size. Colloidal particles have an enormous surface area per unit mass as a result of their small size.
Complete Step By Step Answer:
Colloidal solution is defined as the mixture in which one of the substances disperses into very microscopic particles which are dispersed throughout a second substance. The minute particles are known as colloidal particles. The minute particles are known as colloidal particles. Based on interaction, colloidal solutions can be classified as lyophilic and lyophobic. If the dispersed phase is water these terms are known as hydrophilic and hydrophobic. Hydrophilic colloids are water loving colloids. These colloid particles are attracted toward water. They are also called reversible sols. The stability of a colloidal system is the capability of the system to remain as it is. Stability is hindered by aggregation and sedimentation phenomena, which are driven by the colloid tendency to reduce surface energy Hydrophilic colloids are stable due to a layer of dispersion medium on their particles. The dispersed phase particles have a great affinity for the dispersion medium in lyophilic sols, which are reversible. Examples are gum, gelatin, starch, proteins, etc. The dispersed phase particles have no affinity for the dispersion medium in lyophobic sols, which are irreversible. Examples are solutions of metals such as gold, silver, and metal hydrides.
It is a structure of Micelle. The water-loving (hydrophilic) part of the soap molecules sticks to the water and points outwards, forming the outer surface of the micelle. The oil-loving (hydrophobic) parts stick to the oil and trap oil in the center where it can't come into contact with the water.

Additional Information:
The Colloids are classified based on the following criteria: Physical states of components: this classification is based on the physical state of the dispersed phase and dispersion medium. Depending upon the weather dispersed phase and dispersed medium are solids, liquids, or gases; there are eight types of the colloidal system possible. A gas mixed with other gas forms a homogeneous mixture; hence this system is not a colloidal system. The hydrophilic sols are more stable than hydrophobic sols because the stability of hydrophobic sols is due to charge only and hydrophilic sols are stable due to charge and solvation.
Note :
Remember that Hydrophilic colloids are water loving colloids. These colloid particles are attracted toward water. They are also called reversible sols. The stability of a colloidal system is the capability of the system to remain as it is. Stability is hindered by aggregation and sedimentation phenomena, which are driven by the colloid tendency to reduce surface energy Hydrophilic colloids are stable due to a layer of dispersion medium on their particles.
Complete Step By Step Answer:
Colloidal solution is defined as the mixture in which one of the substances disperses into very microscopic particles which are dispersed throughout a second substance. The minute particles are known as colloidal particles. The minute particles are known as colloidal particles. Based on interaction, colloidal solutions can be classified as lyophilic and lyophobic. If the dispersed phase is water these terms are known as hydrophilic and hydrophobic. Hydrophilic colloids are water loving colloids. These colloid particles are attracted toward water. They are also called reversible sols. The stability of a colloidal system is the capability of the system to remain as it is. Stability is hindered by aggregation and sedimentation phenomena, which are driven by the colloid tendency to reduce surface energy Hydrophilic colloids are stable due to a layer of dispersion medium on their particles. The dispersed phase particles have a great affinity for the dispersion medium in lyophilic sols, which are reversible. Examples are gum, gelatin, starch, proteins, etc. The dispersed phase particles have no affinity for the dispersion medium in lyophobic sols, which are irreversible. Examples are solutions of metals such as gold, silver, and metal hydrides.
It is a structure of Micelle. The water-loving (hydrophilic) part of the soap molecules sticks to the water and points outwards, forming the outer surface of the micelle. The oil-loving (hydrophobic) parts stick to the oil and trap oil in the center where it can't come into contact with the water.

Additional Information:
The Colloids are classified based on the following criteria: Physical states of components: this classification is based on the physical state of the dispersed phase and dispersion medium. Depending upon the weather dispersed phase and dispersed medium are solids, liquids, or gases; there are eight types of the colloidal system possible. A gas mixed with other gas forms a homogeneous mixture; hence this system is not a colloidal system. The hydrophilic sols are more stable than hydrophobic sols because the stability of hydrophobic sols is due to charge only and hydrophilic sols are stable due to charge and solvation.
Note :
Remember that Hydrophilic colloids are water loving colloids. These colloid particles are attracted toward water. They are also called reversible sols. The stability of a colloidal system is the capability of the system to remain as it is. Stability is hindered by aggregation and sedimentation phenomena, which are driven by the colloid tendency to reduce surface energy Hydrophilic colloids are stable due to a layer of dispersion medium on their particles.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




