
Name the process by which nylon \[ - 6,6\] is obtained?
Answer
503.1k+ views
Hint: We need to know that nylon- \[ - 6,6\] is a kind of nylon or it is a polyamide. And these are made up of mainly two monomers. And these monomers contain six carbon atoms. Here each of the polymer chains contains two forms of carbon atoms. The nylon \[ - 6,6\] is a type of synthetic polymer. And this synthetic polymer has good strength and is semi – crystalline. These nylon \[ - 6,6\] are mainly used to prepare cords, carpet etc.
Complete answer:
We need to remember that the nylon\[ - 6,6\]is prepared by using Hexamethylenediamine and adipic acid by condensation polymerization reaction or otherwise known as polycondensation reaction. And this process is a step- growth polymerization. When the Hexamethylenediamine is reacting with adipic acid in the presence of water, there is a formation of nylon\[ - 6,6\]. The adipic acid is a diacid and Hexamethylenediamine is a diamine. And it is a condensation polymer which is obtained by the joining of monomer units by eliminating water molecules. The chemical reaction can be written as,
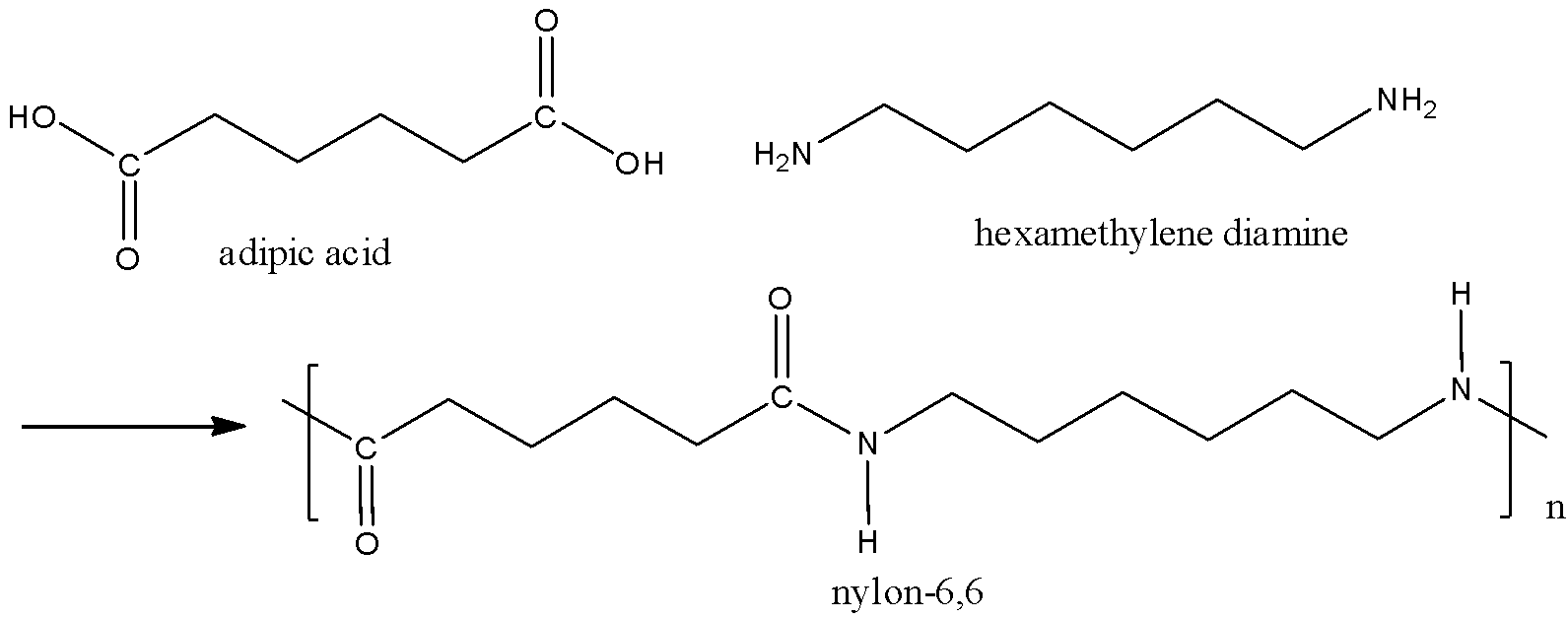
All the nylons have different characters and it is a family of polymers. Therefore two monomers used to make nylon \[ - 6,6\] are adipic acid and Hexamethylenediamine. Each monomer contains six carbon atoms. The melting temperature of nylon\[ - 6,6\]is very high.
Note:
We need to know that the nylon \[ - 6,6\] is prepared by using diacid and diamines and it is adipic acid and Hexamethylenediamine. The nylon \[ - 6,6\] contains a long polymer chain. And this is classified as synthetic polymer. The nylon \[ - 6,6\] is mainly used to make sheets, and in textile industries, and make parachutes etc. And these are waterproof materials. Hence, it is also used to make swimming suits.
Complete answer:
We need to remember that the nylon\[ - 6,6\]is prepared by using Hexamethylenediamine and adipic acid by condensation polymerization reaction or otherwise known as polycondensation reaction. And this process is a step- growth polymerization. When the Hexamethylenediamine is reacting with adipic acid in the presence of water, there is a formation of nylon\[ - 6,6\]. The adipic acid is a diacid and Hexamethylenediamine is a diamine. And it is a condensation polymer which is obtained by the joining of monomer units by eliminating water molecules. The chemical reaction can be written as,

All the nylons have different characters and it is a family of polymers. Therefore two monomers used to make nylon \[ - 6,6\] are adipic acid and Hexamethylenediamine. Each monomer contains six carbon atoms. The melting temperature of nylon\[ - 6,6\]is very high.
Note:
We need to know that the nylon \[ - 6,6\] is prepared by using diacid and diamines and it is adipic acid and Hexamethylenediamine. The nylon \[ - 6,6\] contains a long polymer chain. And this is classified as synthetic polymer. The nylon \[ - 6,6\] is mainly used to make sheets, and in textile industries, and make parachutes etc. And these are waterproof materials. Hence, it is also used to make swimming suits.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




