
Name the functional groups present in the beta form of glucose.
Answer
524.4k+ views
Hint: In order to solve this question, one first needs to know the structure of glucose in its beta form. Glucose is a monosaccharide containing six carbon atoms, hence it’s a hexose. Alpha and beta forms of glucose are two anomeric forms of glucose differing in configuration at the first carbon atom, that is \[C_1\] .
Complete answer:
The structure of beta glucose in Fischer projection is
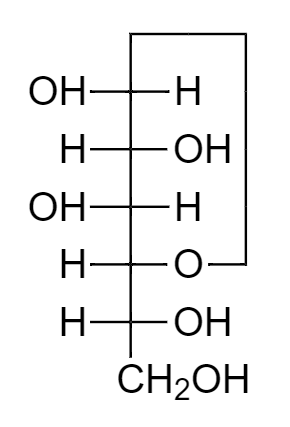
In aqueous solution, glucose molecules majorly exist in the pyranose form, that is, in cyclic isomeric form. This form is created by the cyclisation process between the first and the fifth carbon atom of glucose, leading to a molecule consisting of a six membered stable ring, known as pyranose.
Due to cyclisation, stereoisomers differing in configuration at the first carbon atom are created. They are known as anomers, and are named as alpha glucopyranose and beta glucopyranose.
The structure of beta glucopyranose, the cyclic form of beta glucose is
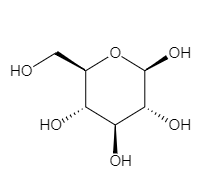
Now, considering the carbon atoms and the functional groups associated with them, it would seem that all carbon atoms are connected to a hydroxyl substituent. However on close observation, we observe that the first carbon and the ether adjacent to it, are forming a hemiacetal. The rest of the carbon atoms all contain the hydroxyl functional group, whereas $C1$ is involved in the formation of a hemiacetal.
Thus, hydroxyl and hemiacetal are the two functional groups present in the beta form of glucose.
Note:
The functional groups of alpha glucose too are hemiacetal and hydroxyl, as the change in configuration does not affect the functional group of a compound. Glucose, as a straight chain compound contains aldehyde and hydroxyl groups as functional groups, however, its cyclisation results in a different set of functional groups.
Complete answer:
The structure of beta glucose in Fischer projection is

In aqueous solution, glucose molecules majorly exist in the pyranose form, that is, in cyclic isomeric form. This form is created by the cyclisation process between the first and the fifth carbon atom of glucose, leading to a molecule consisting of a six membered stable ring, known as pyranose.
Due to cyclisation, stereoisomers differing in configuration at the first carbon atom are created. They are known as anomers, and are named as alpha glucopyranose and beta glucopyranose.
The structure of beta glucopyranose, the cyclic form of beta glucose is

Now, considering the carbon atoms and the functional groups associated with them, it would seem that all carbon atoms are connected to a hydroxyl substituent. However on close observation, we observe that the first carbon and the ether adjacent to it, are forming a hemiacetal. The rest of the carbon atoms all contain the hydroxyl functional group, whereas $C1$ is involved in the formation of a hemiacetal.
Thus, hydroxyl and hemiacetal are the two functional groups present in the beta form of glucose.
Note:
The functional groups of alpha glucose too are hemiacetal and hydroxyl, as the change in configuration does not affect the functional group of a compound. Glucose, as a straight chain compound contains aldehyde and hydroxyl groups as functional groups, however, its cyclisation results in a different set of functional groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




