
What is ‘I’ and name of reaction?
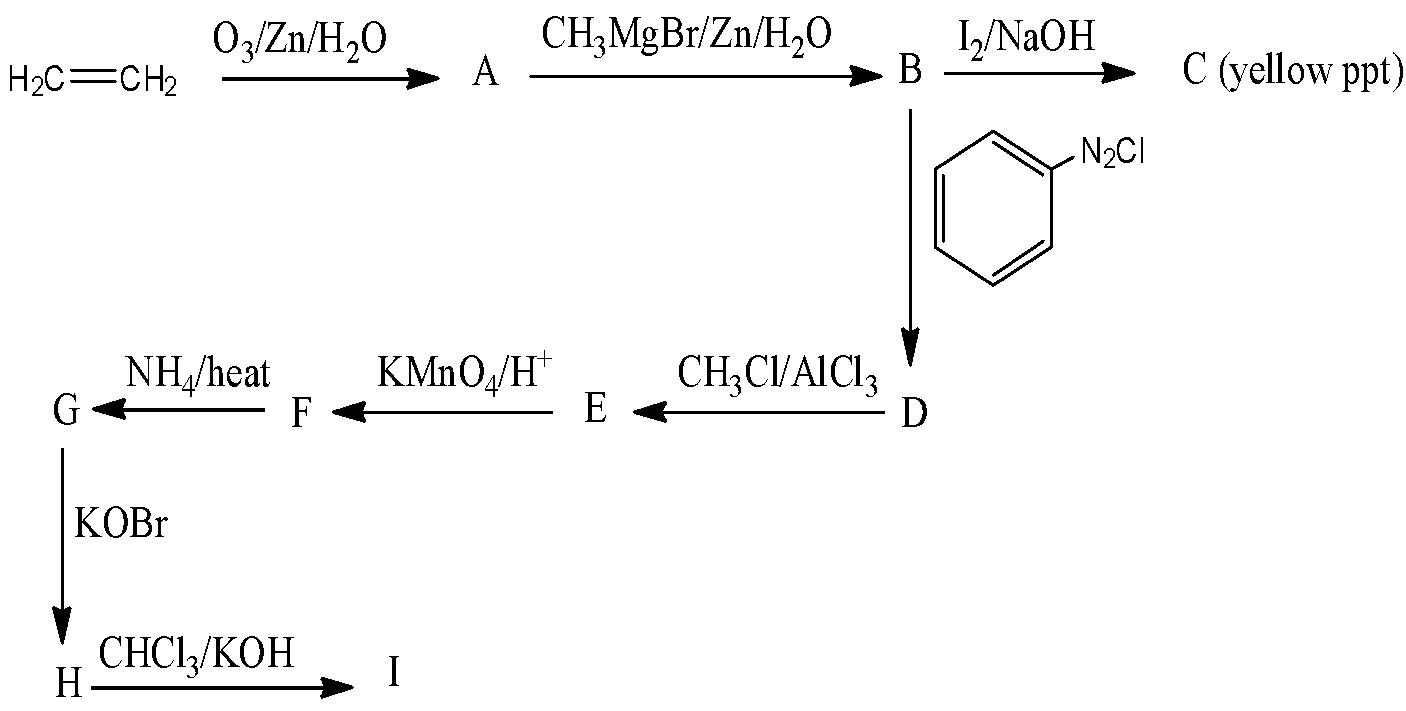
(A) Aniline, Hoffman Bromamide reaction.
(B) Sodium benzoate and Cannizaro reaction
(C) Phenyl isocyanide and Cannizaro
(D) Phenyl isocyanide and Carbylamine test

Answer
548.7k+ views
Hint: In the presence of $Zn$ reductive ozonolysis of alkene takes place and $KMn{{O}_{4}}$ acts as a strong oxidizing agent. Alcohol and carbonyl compounds having three $\alpha $-hydrogen gives iodoform test.
Complete Step by step solution:
In organic chemistry, we have studied the basic organic reactions like oxidation, reduction and so on.
Let us now solve the above question based on this.
-In the presence of$Zn$ozonolysis of alkene leads to incomplete oxidation and forms two molecules of formic acid. The reaction is,
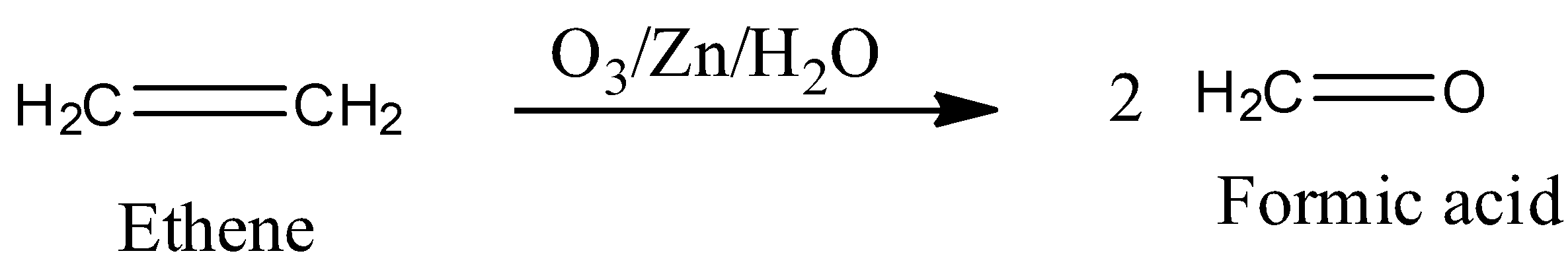
The reaction of this formic acid with Grignard reagent leads to nucleophilic addition of methyl group and as a result forms ethyl alcohol.
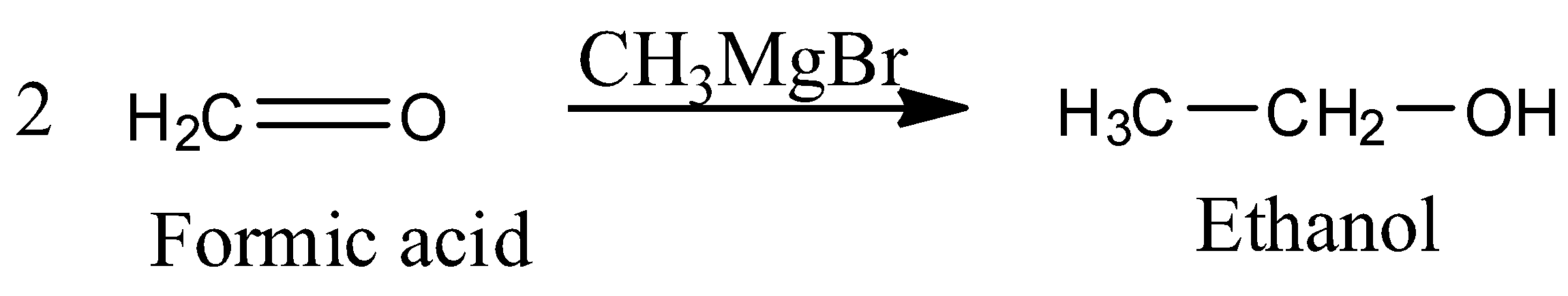
Now, in iodoform reaction oxidation and reduction, both take place, as a result, iodoform a yellow coloured compound is formed. The reaction between ethyl alcohol and iodine in the presence of $NaOH$ gives iodoform test
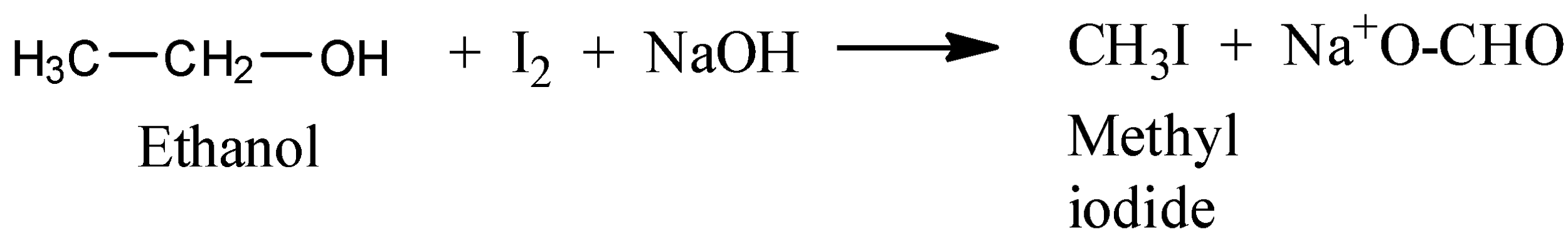
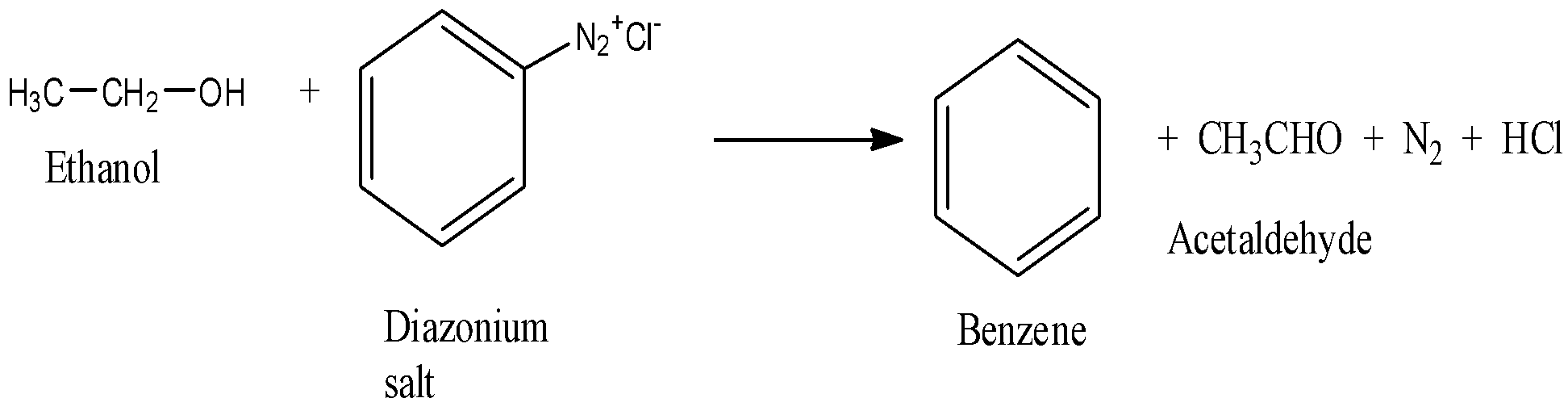
The reaction in between ethyl alcohol and benzene diazonium salt leads to the reduction of benzene diazonium salt to benzene and ethyl alcohol get oxidized into acetaldehyde.
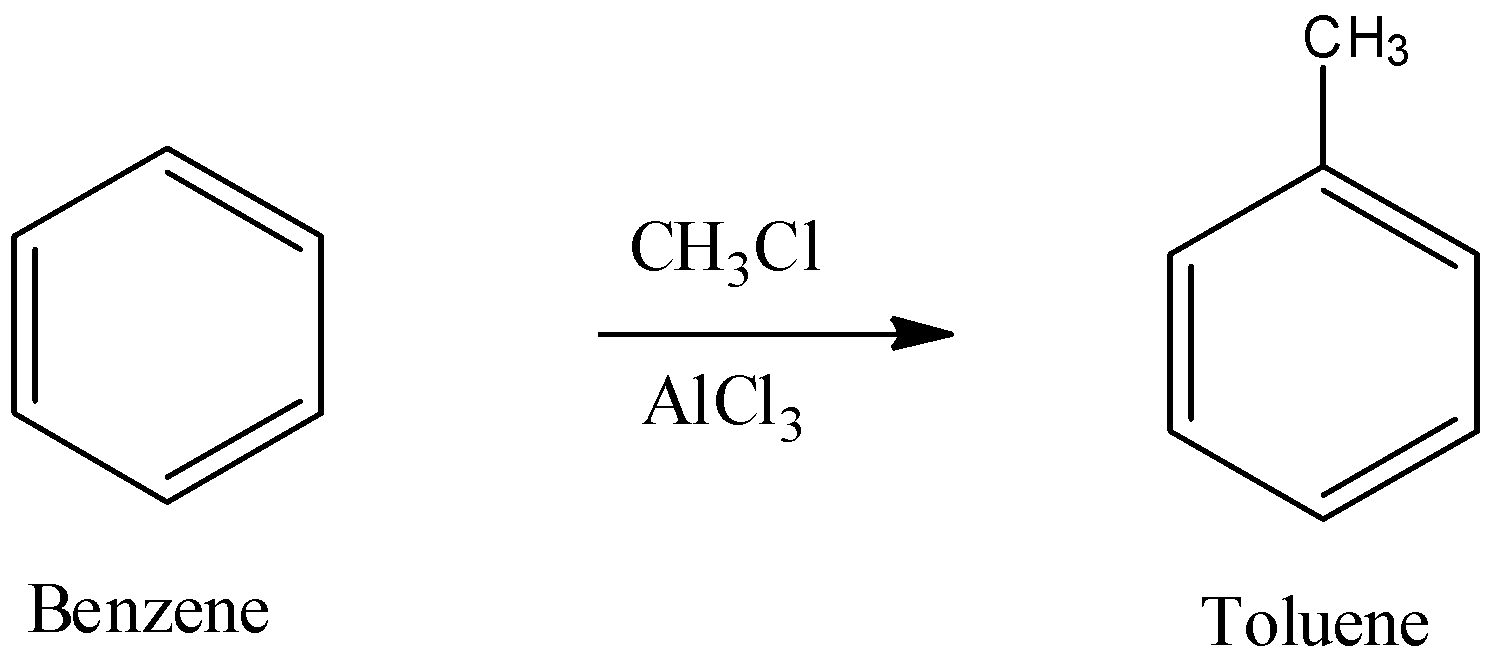
The reaction in between benzene and chloroform in the presence of $AlC{{l}_{3}}$ causes electrophilic addition of $-C{{H}_{3}}$ group and form toluene. This reaction is known as Friedel-Crafts alkylation reaction.
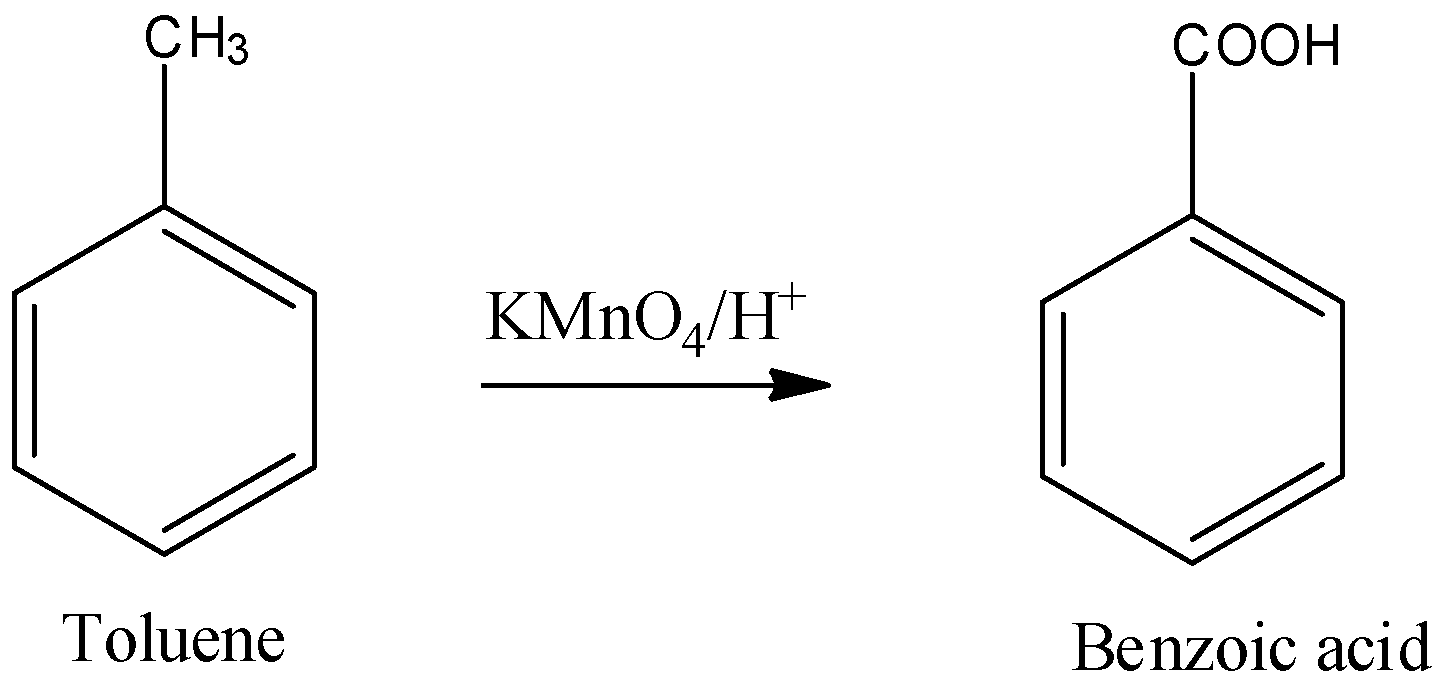
Oxidation of toluene takes place in the presence of $KMn{{O}_{4}}$ which produces benzoic acid.
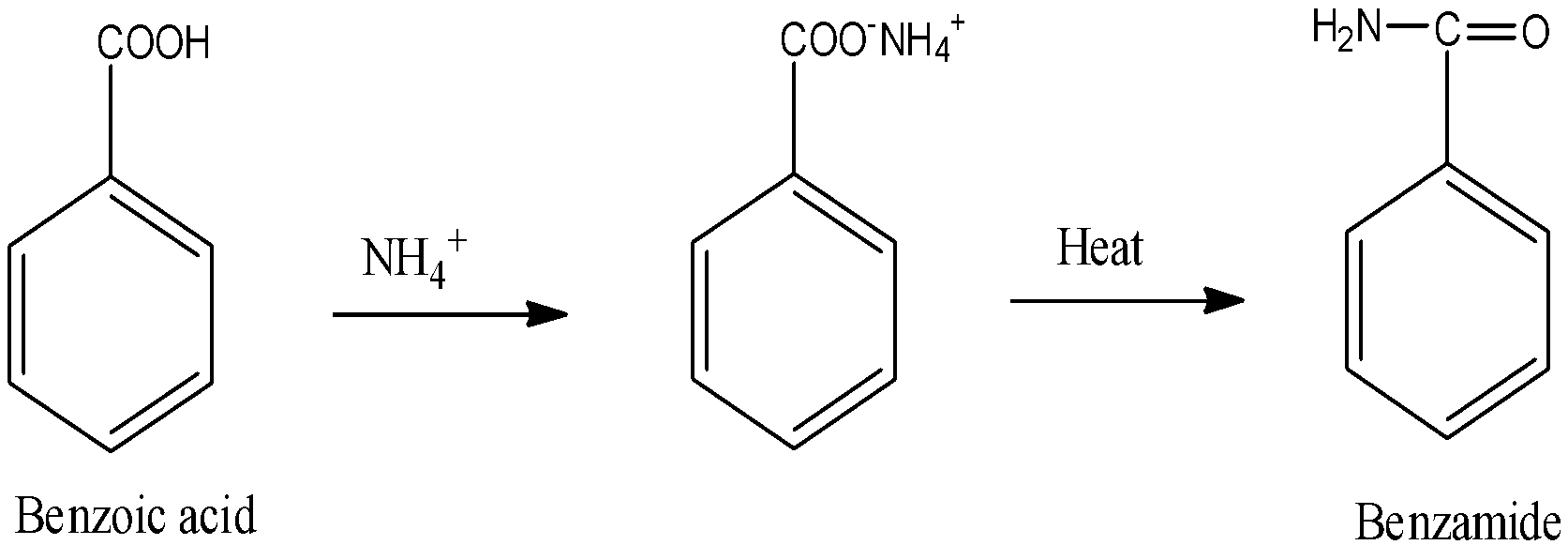
The acid-base reaction takes place in between benzoic acid and ammonium as a result ammonium salt of benzoic will form. On heating this salt of benzoic acid produces benzamide.
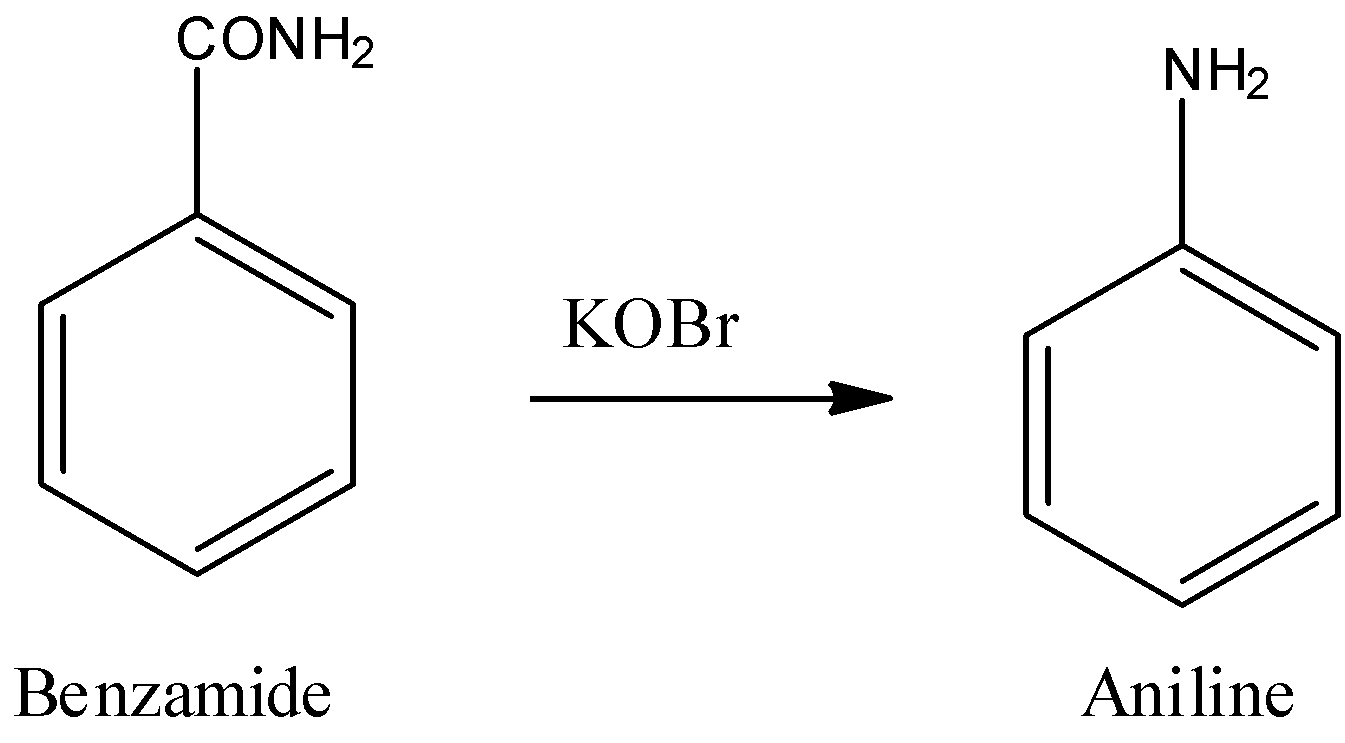
When benzamide is treated with a mixture of $B{{r}_{2}}$ in the presence of $KOH$, it will form aniline this reaction is known as Hoffmann’s Bromamide reaction.
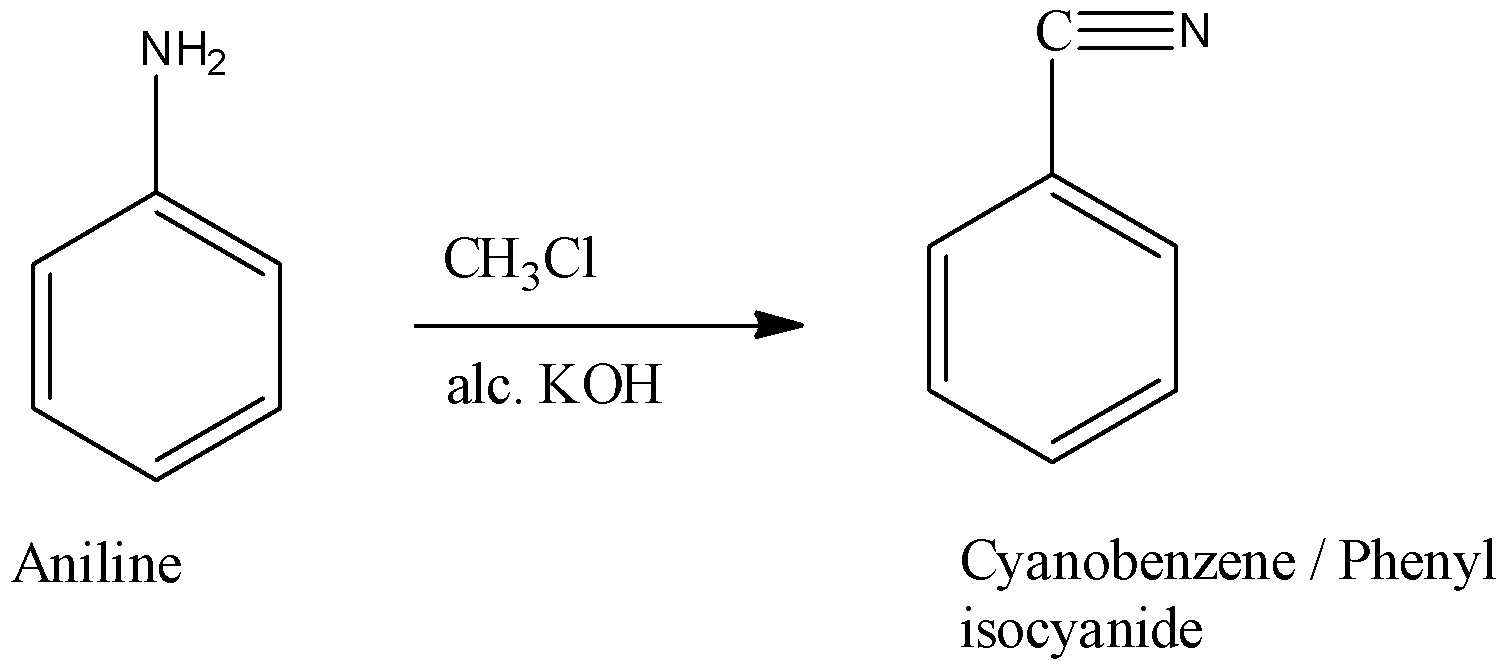
Aromatic primary amine (aniline) is heated with chloroform in the presence of alcoholic $KOH$ it will produce isocyanide this reaction is known as carbylamine reaction.
So, the option (D) is the correct answer.
Note: Carbylamine reaction is used as a test for primary amine. Aniline being a primary amine gives carbylamines test. Cannizaro reaction is the reaction of aldehyde in the presence of a strong base in which one molecule of aldehyde is oxidized and other molecules will reduce.
Complete Step by step solution:
In organic chemistry, we have studied the basic organic reactions like oxidation, reduction and so on.
Let us now solve the above question based on this.
-In the presence of$Zn$ozonolysis of alkene leads to incomplete oxidation and forms two molecules of formic acid. The reaction is,
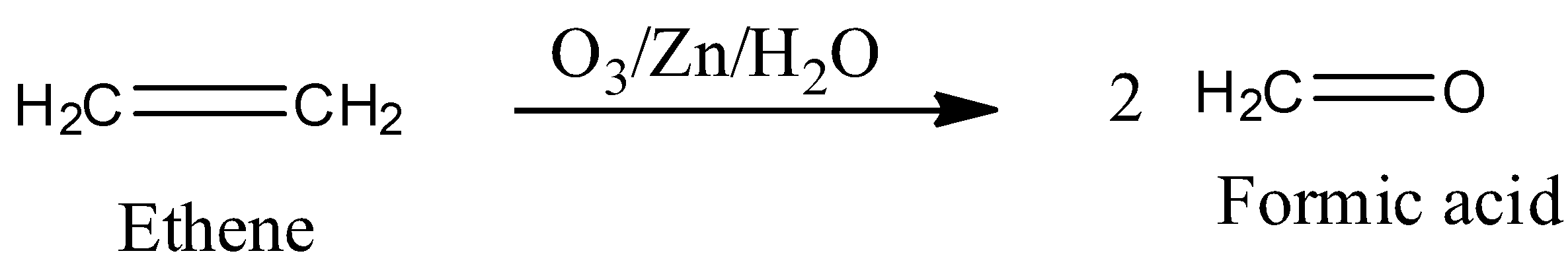
The reaction of this formic acid with Grignard reagent leads to nucleophilic addition of methyl group and as a result forms ethyl alcohol.
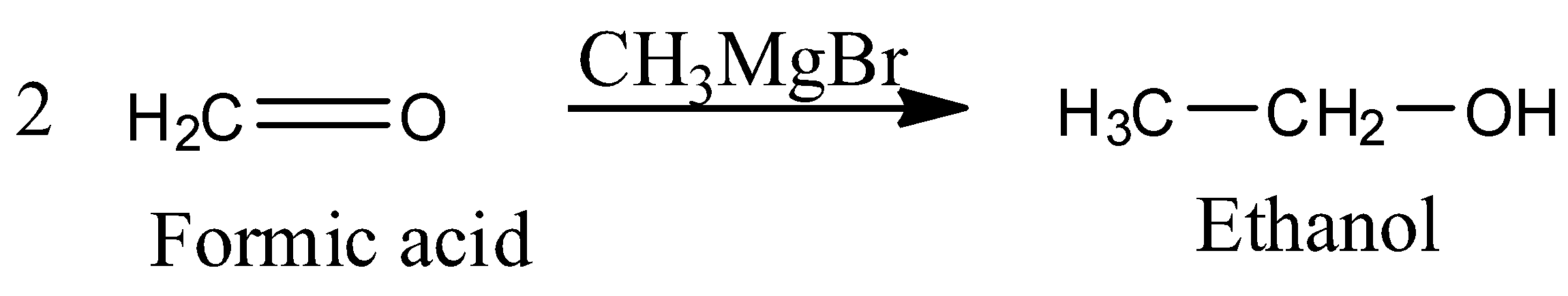
Now, in iodoform reaction oxidation and reduction, both take place, as a result, iodoform a yellow coloured compound is formed. The reaction between ethyl alcohol and iodine in the presence of $NaOH$ gives iodoform test
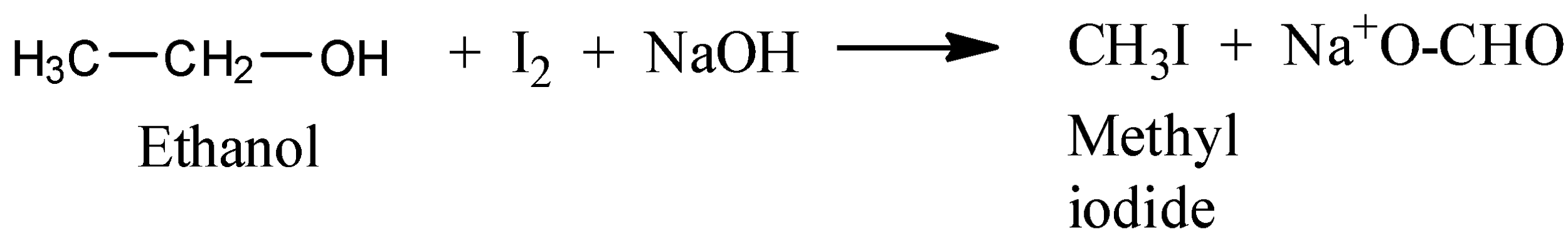
The reaction in between ethyl alcohol and benzene diazonium salt leads to the reduction of benzene diazonium salt to benzene and ethyl alcohol get oxidized into acetaldehyde.

The reaction in between benzene and chloroform in the presence of $AlC{{l}_{3}}$ causes electrophilic addition of $-C{{H}_{3}}$ group and form toluene. This reaction is known as Friedel-Crafts alkylation reaction.

Oxidation of toluene takes place in the presence of $KMn{{O}_{4}}$ which produces benzoic acid.

The acid-base reaction takes place in between benzoic acid and ammonium as a result ammonium salt of benzoic will form. On heating this salt of benzoic acid produces benzamide.

When benzamide is treated with a mixture of $B{{r}_{2}}$ in the presence of $KOH$, it will form aniline this reaction is known as Hoffmann’s Bromamide reaction.

Aromatic primary amine (aniline) is heated with chloroform in the presence of alcoholic $KOH$ it will produce isocyanide this reaction is known as carbylamine reaction.

So, the option (D) is the correct answer.
Note: Carbylamine reaction is used as a test for primary amine. Aniline being a primary amine gives carbylamines test. Cannizaro reaction is the reaction of aldehyde in the presence of a strong base in which one molecule of aldehyde is oxidized and other molecules will reduce.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




