
Most stable lewis structure of ${N_2}O$ is:
A:
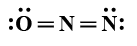
B:
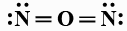
C:
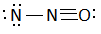
D:
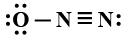
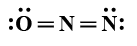
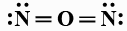
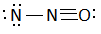
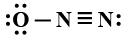
Answer
592.8k+ views
Hint: To find the structure of molecules we must know the electronic configuration of the atoms that molecules possess. Valence electrons are used in making the structure of molecules. Valence electrons are the electrons that are present in the outermost shell of an atom.
Complete step by step answer:
In this question, we have to find the most stable structure of ${N_2}O$. The structure will be stable if there will be a negative charge on more electronegative elements. Among nitrogen and oxygen, oxygen is more electronegative than nitrogen so the structure in which there will be a negative charge on nitrogen will be most stable. Among given options option D is the only option in which there is a negative charge on oxygen (usually it makes two bonds but there is only one bond) and a positive charge on the central nitrogen atom (usually nitrogen forms three bonds but central nitrogen has four bonds one with oxygen and three with another nitrogen atom). charges on the structure will look like:
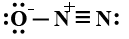
So correct answer is option D that is
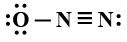
Additional information: ${N_2}O$ is nitrogen dioxide. It is one of the oxides of nitrogen. It is an intermediate formed during the synthesis of nitric acid. It is widely used in the production of fertilizers. ${N_2}O$is among primary pollutants. Pollutants are the substances which cause pollution.
Note:
Resonance structures are sets of lewis structures. These structures describe the delocalization of electrons in an ion or a molecule. These structures are also called canonical structures.
Complete step by step answer:
In this question, we have to find the most stable structure of ${N_2}O$. The structure will be stable if there will be a negative charge on more electronegative elements. Among nitrogen and oxygen, oxygen is more electronegative than nitrogen so the structure in which there will be a negative charge on nitrogen will be most stable. Among given options option D is the only option in which there is a negative charge on oxygen (usually it makes two bonds but there is only one bond) and a positive charge on the central nitrogen atom (usually nitrogen forms three bonds but central nitrogen has four bonds one with oxygen and three with another nitrogen atom). charges on the structure will look like:
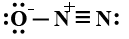
So correct answer is option D that is
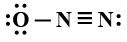
Additional information: ${N_2}O$ is nitrogen dioxide. It is one of the oxides of nitrogen. It is an intermediate formed during the synthesis of nitric acid. It is widely used in the production of fertilizers. ${N_2}O$is among primary pollutants. Pollutants are the substances which cause pollution.
Note:
Resonance structures are sets of lewis structures. These structures describe the delocalization of electrons in an ion or a molecule. These structures are also called canonical structures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




