
How many monochlorinated products, (including stereoisomers) may be obtained when an alkane shown below is heated in the presence of \[C{l_2}\]?
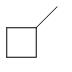
A. 2
B. 4
C. 5
D. 6
E. 8

Answer
609.6k+ views
Hint: Monochlorination as its name suggests refers to chlorination with a single atom even in cases where multiple chlorinations are possible. To find the number of monochlorinated products, the only way is to find the number of structures drawn.
Complete answer:
Monochlorination of an alkane involves substituting one of the hydrogen in the alkane with a chlorine atom. This is achieved by treating the alkane with chlorine in the presence of UV light. These conditions cause the chlorine molecule to split into chlorine free radicals.
We must remember when alkanes larger than ethane are halogenated, isomeric products are formed. Thus chlorination of propane gives both 1-chloropropane and 2-chloropropane as mono-chlorinated products.
Let us first have a look at the chlorination mechanism:
Firstly, the chlorine molecule splits to two chlorine atoms in presence of UV rays or sunlight. Each atom can now act as a free radical.
On interacting with alkane, hydrogen atom bonds with a free radical forming a primary methyl radical.
Hence, we get the desired product and a chlorine radical to accompany.
In cases of light alkanes, all hydrogen atoms are equivalent hence all of them have the same probability to be replaced. But for higher alkanes, the hydrogen atoms which form a part of \[C{H_2}\]groups are preferentially selected.
Keeping this mechanism in mind, we would now draw all possible structures pertaining to the question.
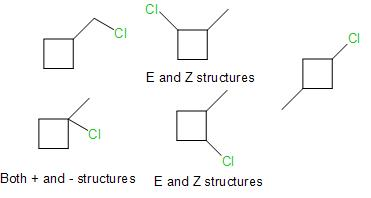
On counting the number of structures including the stereoisomers, we end up with 8 structures in total.
Hence, the correct answer is Option (E) 8.
Note: Most of the time we should know that halogenation does not end with just a monosubstituted product. The chlorination of methane yields dichloromethane, chloroform and carbon tetrachloride. But we can predict the product distribution of different monochloro derivatives resulting from the chlorination of an alkane with non-equivalent hydrogen.
Complete answer:
Monochlorination of an alkane involves substituting one of the hydrogen in the alkane with a chlorine atom. This is achieved by treating the alkane with chlorine in the presence of UV light. These conditions cause the chlorine molecule to split into chlorine free radicals.
We must remember when alkanes larger than ethane are halogenated, isomeric products are formed. Thus chlorination of propane gives both 1-chloropropane and 2-chloropropane as mono-chlorinated products.
Let us first have a look at the chlorination mechanism:
Firstly, the chlorine molecule splits to two chlorine atoms in presence of UV rays or sunlight. Each atom can now act as a free radical.
On interacting with alkane, hydrogen atom bonds with a free radical forming a primary methyl radical.
Hence, we get the desired product and a chlorine radical to accompany.
In cases of light alkanes, all hydrogen atoms are equivalent hence all of them have the same probability to be replaced. But for higher alkanes, the hydrogen atoms which form a part of \[C{H_2}\]groups are preferentially selected.
Keeping this mechanism in mind, we would now draw all possible structures pertaining to the question.

On counting the number of structures including the stereoisomers, we end up with 8 structures in total.
Hence, the correct answer is Option (E) 8.
Note: Most of the time we should know that halogenation does not end with just a monosubstituted product. The chlorination of methane yields dichloromethane, chloroform and carbon tetrachloride. But we can predict the product distribution of different monochloro derivatives resulting from the chlorination of an alkane with non-equivalent hydrogen.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




