
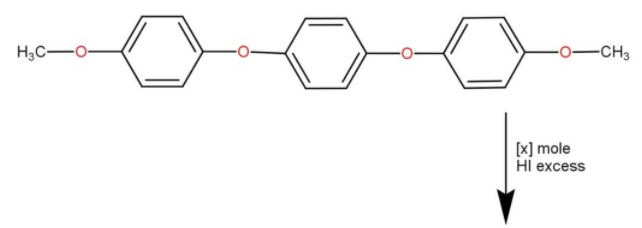
How many moles of $HI$ will be consumed in the given reaction?
A. 3 moles
B. 2 moles
C. 1 mole
D. 4 moles

Answer
568.8k+ views
Hint:We can reduce carbonyl compounds and esters to alcohol using strong reagents like $LiA{H_4}$ or $HI$. If the reducing agent is mild like HI, then alcohol is formed. If the reducing agent is strong like Zn/HCl, then the carbonyl group is reduced to hydrocarbons.
Complete answer:
We know that ethers are compounds with ${\text{R}} - {\text{O}} - {\text{R}}$ structure, where R is some alkyl group.In the question, we can see that it has two ${\text{R}} - {\text{O}} - {\text{R}}$ structure at both endings. Also, we are aware of the fact that $HI$ is a strong reducing agent. When we reduce esters we get alcohol. This is the properties of ester as well as the preparation of alcohols. For preparation, we use $LiA{H_4}$ since they are stronger reducing agents than $HI$.We know that in ${\text{R}} - {\text{O}} - {\text{R}}$ there are two lone pairs in hydrogen making them highly reactive toward acidic substances.
The $HI$ dissociates into ions as shown below
$HI \to {H^ + } + {I^ - }$
The ${H^ + }$ produced will reactivate the lone pairs of O-atoms in ethers. When considering our question we have a total of 4 oxygen atoms. The last two oxygen atoms are attached to the $ - C{H_3}$ group. Those will have less steric hindrance to them making them more reactive towards $HI$
Now we will discuss what is meant by steric effect or steric hindrance, it happens when a large sized group prevents molecules from the chemical reaction happening. Since we have a large phenyl group attached to two of the oxygen atoms, it is less likely to get reacted with strong reducing agents. The last two of them will get reacted to $HI$ and the ${I^ - }$ will combine with the large phenyl part at the ${H^ + }$ will combine with $O - {\text{C}}{{\text{H}}_3}$ to form alcohol. This will happen at the both ends, hence we need two moles of $HI$
i.e., option B is correct
Note:
At first it may occur like 4 moles is required, but here we must not forget to consider the steric effect. If we do not consider it may look like we need 4 moles, which is wrong.
Complete answer:
We know that ethers are compounds with ${\text{R}} - {\text{O}} - {\text{R}}$ structure, where R is some alkyl group.In the question, we can see that it has two ${\text{R}} - {\text{O}} - {\text{R}}$ structure at both endings. Also, we are aware of the fact that $HI$ is a strong reducing agent. When we reduce esters we get alcohol. This is the properties of ester as well as the preparation of alcohols. For preparation, we use $LiA{H_4}$ since they are stronger reducing agents than $HI$.We know that in ${\text{R}} - {\text{O}} - {\text{R}}$ there are two lone pairs in hydrogen making them highly reactive toward acidic substances.
The $HI$ dissociates into ions as shown below
$HI \to {H^ + } + {I^ - }$
The ${H^ + }$ produced will reactivate the lone pairs of O-atoms in ethers. When considering our question we have a total of 4 oxygen atoms. The last two oxygen atoms are attached to the $ - C{H_3}$ group. Those will have less steric hindrance to them making them more reactive towards $HI$
Now we will discuss what is meant by steric effect or steric hindrance, it happens when a large sized group prevents molecules from the chemical reaction happening. Since we have a large phenyl group attached to two of the oxygen atoms, it is less likely to get reacted with strong reducing agents. The last two of them will get reacted to $HI$ and the ${I^ - }$ will combine with the large phenyl part at the ${H^ + }$ will combine with $O - {\text{C}}{{\text{H}}_3}$ to form alcohol. This will happen at the both ends, hence we need two moles of $HI$
i.e., option B is correct
Note:
At first it may occur like 4 moles is required, but here we must not forget to consider the steric effect. If we do not consider it may look like we need 4 moles, which is wrong.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




