
How many moles of $C{{H}_{3}}OH$ required to react completely with B?
(A) One
(B) Two
(C) Three
(D) Four

Answer
591.6k+ views
Hint: Chlorine gas in presence of light, halogenated the alkyl group by substituting H-atom bonded with carbon of alkyl group. NaOH contains hydroxide ions and it can attack relatively electropositive carbon atoms.
Complete step by step answer:
To find the number of moles of \[C{{H}_{3}}OH\] required, we have to first find the product B. Then we can find the number of moles.
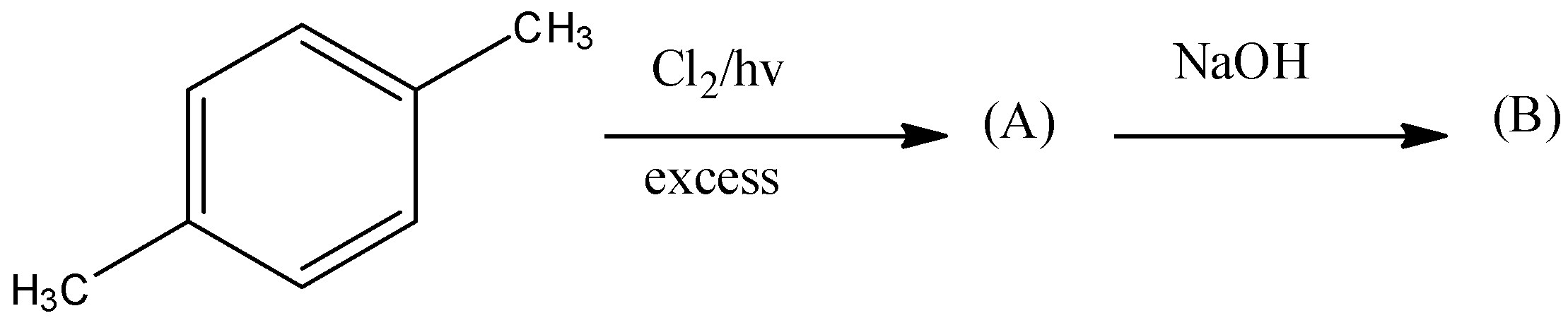
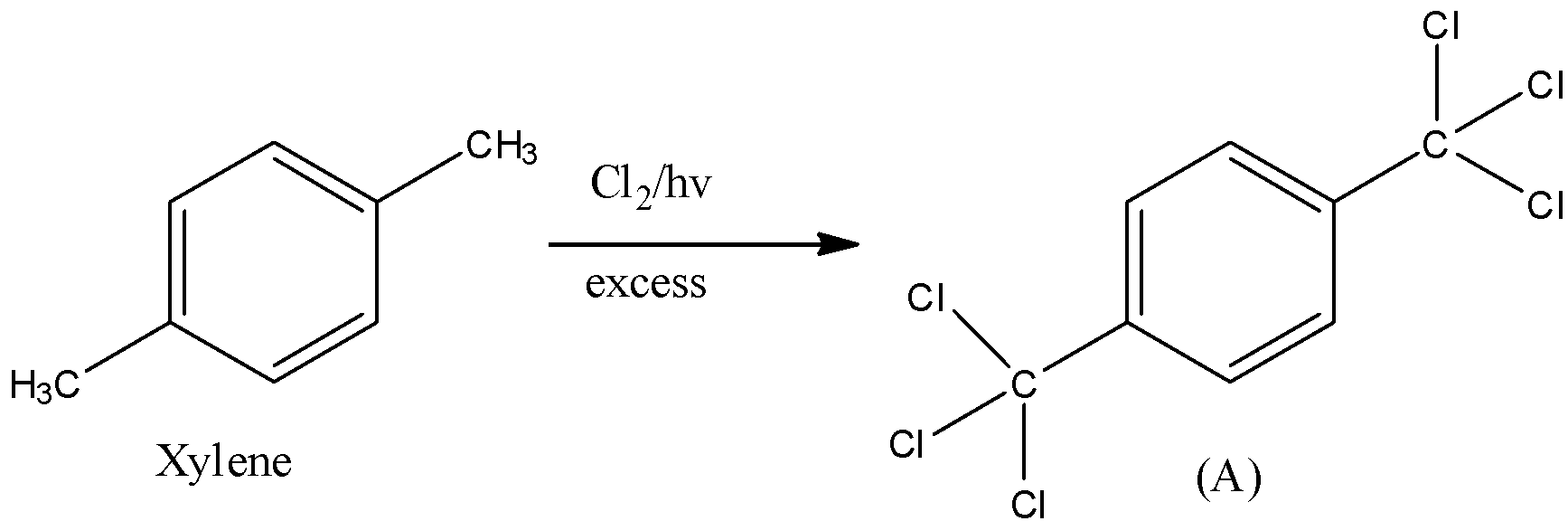
First we will take the first part of the reaction. In this part all the atoms of hydrogen on xylene will get replaced by chlorine. Chlorine gas in presence of light always substitutes hydrogen atoms of the alkyl group. When excess chlorine gas is used, all the hydrogen atoms will get replaced with chlorine atoms. So, we will get hexachloro compound as a product of this step.
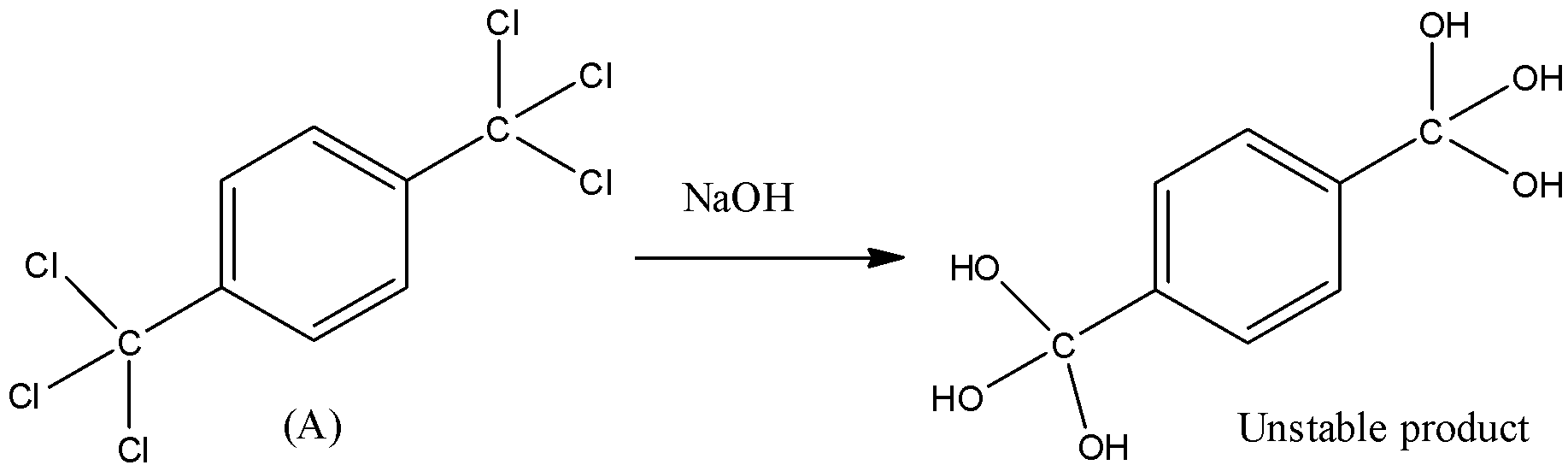
This structure is highly reactive, and it can be easily hydrolysed by NaOH. So, hydroxide ions will attack the carbon atom having chlorine atoms and chlorine atoms will get removed one by one to give a compound having six hydroxyl groups.
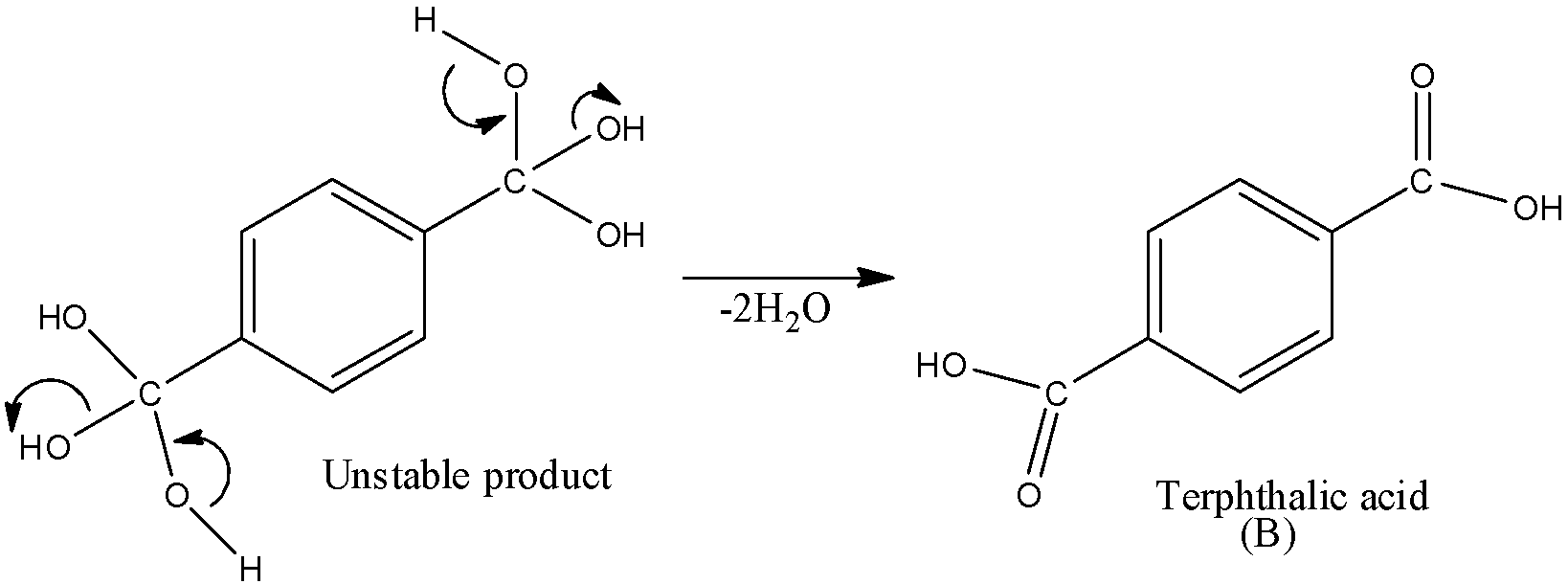
We should note that B’ is not the final product, dehydration will occur and this will transform into acid.
So, B is the final product which is benzene-1,4-dicarboxylic acid.
Now, we will come to the second part of our question : we have to find the number of moles of $C{{H}_{3}}OH$ , that is required to react with benzene-1,4-dicarboxylic acid.
We should know that acid in the presence of alcohol gives a product ester which is known by process esterification. One mole of alcohol is required for one mole of acid to give one mole of ester product. Here, we can see that benzene-1,4-dicarboxylic acid contains two –COOH groups. So, it will require two moles of alcohol to produce a di-ester. We can write the reaction of compound (B) with methanol as under.
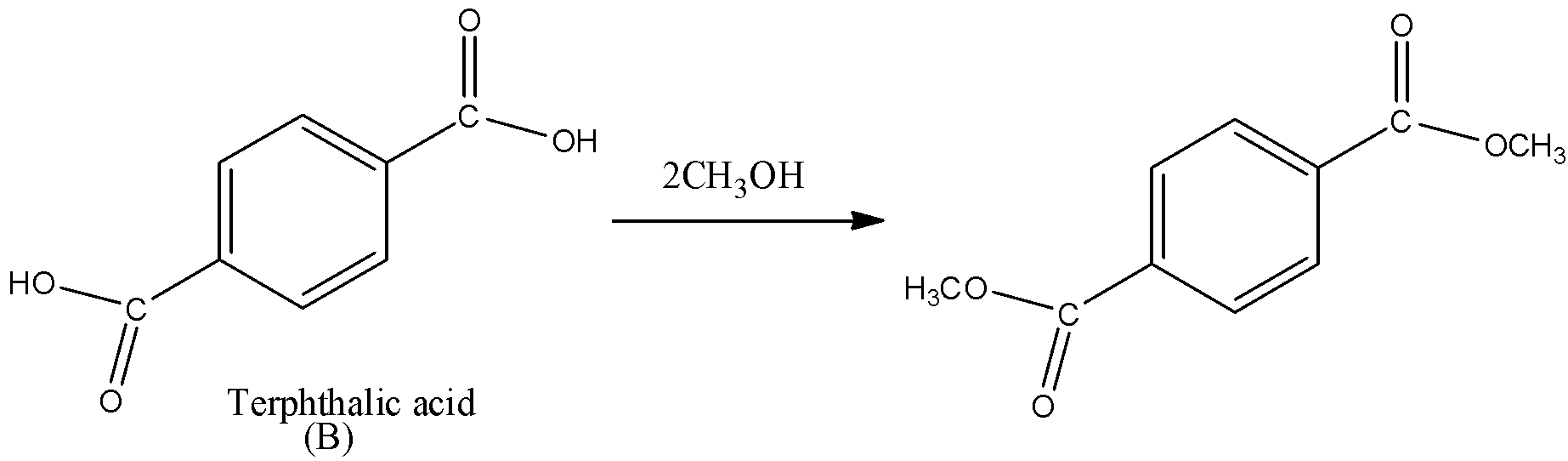
So, from this we can say that our answer is two moles of $C{{H}_{3}}OH$
So, the correct answer is “Option B”.
Note: Remember that in the first step, the hydrogen atoms of benzene ring cannot be substituted by chlorine atoms. It requires different conditions to replace them. Remember that chlorination of alkyl groups is a step by step process but when gas is used in excess amount, it will halogenate all possible places.
Complete step by step answer:
To find the number of moles of \[C{{H}_{3}}OH\] required, we have to first find the product B. Then we can find the number of moles.
First we will take the first part of the reaction. In this part all the atoms of hydrogen on xylene will get replaced by chlorine. Chlorine gas in presence of light always substitutes hydrogen atoms of the alkyl group. When excess chlorine gas is used, all the hydrogen atoms will get replaced with chlorine atoms. So, we will get hexachloro compound as a product of this step.

This structure is highly reactive, and it can be easily hydrolysed by NaOH. So, hydroxide ions will attack the carbon atom having chlorine atoms and chlorine atoms will get removed one by one to give a compound having six hydroxyl groups.

We should note that B’ is not the final product, dehydration will occur and this will transform into acid.
So, B is the final product which is benzene-1,4-dicarboxylic acid.

Now, we will come to the second part of our question : we have to find the number of moles of $C{{H}_{3}}OH$ , that is required to react with benzene-1,4-dicarboxylic acid.
We should know that acid in the presence of alcohol gives a product ester which is known by process esterification. One mole of alcohol is required for one mole of acid to give one mole of ester product. Here, we can see that benzene-1,4-dicarboxylic acid contains two –COOH groups. So, it will require two moles of alcohol to produce a di-ester. We can write the reaction of compound (B) with methanol as under.

So, from this we can say that our answer is two moles of $C{{H}_{3}}OH$
So, the correct answer is “Option B”.
Note: Remember that in the first step, the hydrogen atoms of benzene ring cannot be substituted by chlorine atoms. It requires different conditions to replace them. Remember that chlorination of alkyl groups is a step by step process but when gas is used in excess amount, it will halogenate all possible places.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




