
What is the molar mass of carbon tetrachloride?
Answer
532.2k+ views
Hint :The molar mass of any chemical compound refers to the ratio of mass of a sample of that particular compound and the amount of substance in that particular sample (in moles). The molar mass is actually a bulk characteristic of a substance rather than molecular. The molar mass is generally represented as $ gmo{l^{ - 1}} $ .
Complete Step By Step Answer:
The molar mass of any compound can be found out by adding the relative atomic masses of each element present in that particular compound. The number of atoms in a compound can be determined from their chemical formula.
Now, let us calculate the molar mass of the given compound i.e. carbon tetrachloride. The chemical formula of carbon tetrachloride is $ CC{l_4} $ while its structure is depicted below:
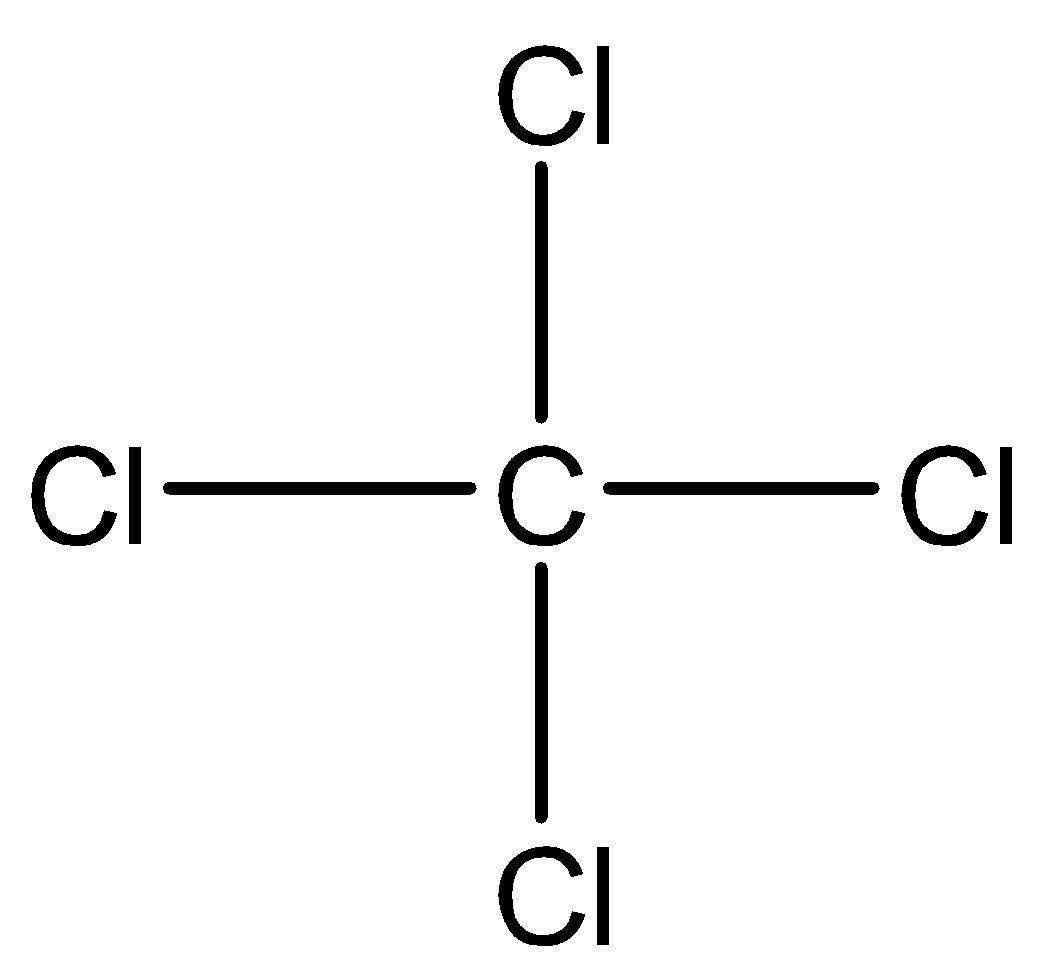
We already know the atomic mass of carbon and chlorine which is mentioned below:
$ C = 12.0107\;u \\
Cl = 35.453\;u \\ $
From the chemical formula and structure, it is clear that carbon tetrachloride comprises of one atom of carbon and four atoms of chlorine and thus molar mass of this compound can be easily calculated by adding the mass of one carbon atoms and four chlorine atoms as shown below:
$ Molar{\text{ }}mass{\text{ }}of{\text{ }}CC{l_4} = (1 \times C) + (4 \times Cl) \\
= (1 \times 12.0107) + (4 \times 35.453) = 153.8227gmo{l^{ - 1}} \\ $
Hence, the molar mass of $ CC{l_4} $ is 153.8227 $ gmo{l^{ - 1}} $ .
Note :
Molar mass plays a significant role in chemistry especially during setting up an experiment. During testing principles which involve specific amounts or quantities of a substance, molar mass is used to figure out the exact quantity to be weighed of that particular substance. Basically molar mass is used to determine the stoichiometry in the chemical reactions as well as equations.
Complete Step By Step Answer:
The molar mass of any compound can be found out by adding the relative atomic masses of each element present in that particular compound. The number of atoms in a compound can be determined from their chemical formula.
Now, let us calculate the molar mass of the given compound i.e. carbon tetrachloride. The chemical formula of carbon tetrachloride is $ CC{l_4} $ while its structure is depicted below:

We already know the atomic mass of carbon and chlorine which is mentioned below:
$ C = 12.0107\;u \\
Cl = 35.453\;u \\ $
From the chemical formula and structure, it is clear that carbon tetrachloride comprises of one atom of carbon and four atoms of chlorine and thus molar mass of this compound can be easily calculated by adding the mass of one carbon atoms and four chlorine atoms as shown below:
$ Molar{\text{ }}mass{\text{ }}of{\text{ }}CC{l_4} = (1 \times C) + (4 \times Cl) \\
= (1 \times 12.0107) + (4 \times 35.453) = 153.8227gmo{l^{ - 1}} \\ $
Hence, the molar mass of $ CC{l_4} $ is 153.8227 $ gmo{l^{ - 1}} $ .
Note :
Molar mass plays a significant role in chemistry especially during setting up an experiment. During testing principles which involve specific amounts or quantities of a substance, molar mass is used to figure out the exact quantity to be weighed of that particular substance. Basically molar mass is used to determine the stoichiometry in the chemical reactions as well as equations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




