
Methyl D- glucoside on reaction with $HI{O_4}$consumes two moles of reagent and produces the dialdehyde $A$ and mole of $HCOOH$. The result of this reaction proves that glucose has pyranose structure.
Answer
585k+ views
Hint: Saccharides: The unit structure of carbohydrates, are known as saccharides.
Pyranose structure: The saccharides which have six membered rings containing five carbon and one oxygen atom, are known as pyranose structure.
Complete step by step answer:
First of all we will discuss carbohydrates, saccharides, structures.
Carbohydrates: The sugar, starch and fibres found in fruits and vegetables, are known as carbohydrates. A carbohydrate is a molecule consisting of carbon, hydrogen and oxygen with hydrogen and oxygen ratio as $2:1$. So its empirical formula is as ${C_m}{({H_2}O)_n}$.
Saccharides: The unit structure by which carbohydrates are formed, known as saccharides. Saccharides are divided into four groups as: monosaccharide, disaccharide, oligosaccharide and polysaccharide.
Monosaccharide: These are the simplest carbohydrates which cannot be hydrolysed into smaller carbohydrates. For example: glucose, fructose.
Disaccharide: Two monosaccharides are joined together to form disaccharides. They are linked together by a covalent bond, known as glycosidic linkage. For example: sucrose, lactose.
Oligosaccharide:Carbohydrates containing three-ten molecules of saccharide, is known as oligosaccharide. For example: Raffinose
Polysaccharide: Carbohydrates containing more than ten molecules of saccharides, are known as polysaccharide. For example: starch.
Pyranose structure: The saccharides which have six membered rings containing five carbon and one oxygen atom, are known as pyranose structure.
Furanose structure: The saccharides which have five membered rings containing four carbon and one oxygen atom, are known as pyranose structure.
The molecular formula of glucose is ${C_6}{H_{12}}{O_6}$. The structure of glucose is pyranose which has six membered rings containing five carbon and one oxygen.
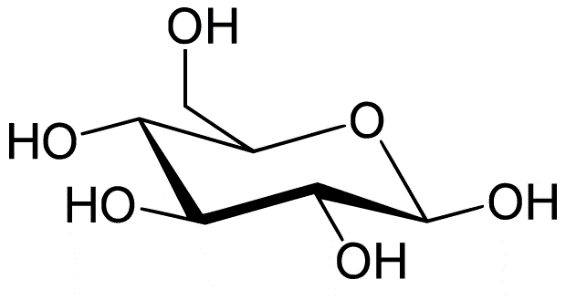
The reaction is as follows:
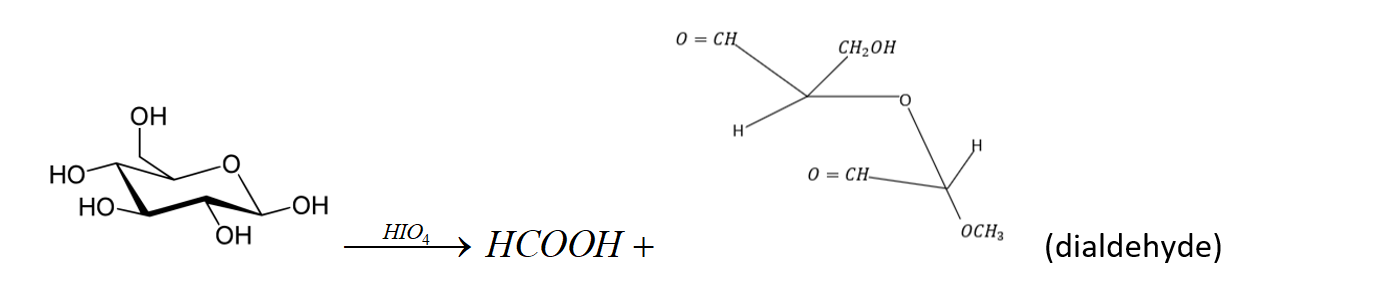
Note:
Chiral carbon: Those carbons whose all the four valencies are filled by different atoms or molecules. D- and L- configuration are different. It is based on the position of the hydroxyl group present in the carbohydrate.
Pyranose structure: The saccharides which have six membered rings containing five carbon and one oxygen atom, are known as pyranose structure.
Complete step by step answer:
First of all we will discuss carbohydrates, saccharides, structures.
Carbohydrates: The sugar, starch and fibres found in fruits and vegetables, are known as carbohydrates. A carbohydrate is a molecule consisting of carbon, hydrogen and oxygen with hydrogen and oxygen ratio as $2:1$. So its empirical formula is as ${C_m}{({H_2}O)_n}$.
Saccharides: The unit structure by which carbohydrates are formed, known as saccharides. Saccharides are divided into four groups as: monosaccharide, disaccharide, oligosaccharide and polysaccharide.
Monosaccharide: These are the simplest carbohydrates which cannot be hydrolysed into smaller carbohydrates. For example: glucose, fructose.
Disaccharide: Two monosaccharides are joined together to form disaccharides. They are linked together by a covalent bond, known as glycosidic linkage. For example: sucrose, lactose.
Oligosaccharide:Carbohydrates containing three-ten molecules of saccharide, is known as oligosaccharide. For example: Raffinose
Polysaccharide: Carbohydrates containing more than ten molecules of saccharides, are known as polysaccharide. For example: starch.
Pyranose structure: The saccharides which have six membered rings containing five carbon and one oxygen atom, are known as pyranose structure.
Furanose structure: The saccharides which have five membered rings containing four carbon and one oxygen atom, are known as pyranose structure.
The molecular formula of glucose is ${C_6}{H_{12}}{O_6}$. The structure of glucose is pyranose which has six membered rings containing five carbon and one oxygen.

The reaction is as follows:

Note:
Chiral carbon: Those carbons whose all the four valencies are filled by different atoms or molecules. D- and L- configuration are different. It is based on the position of the hydroxyl group present in the carbohydrate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




