
How is methyl cyanide obtained from acetamide?
Answer
481.5k+ views
Hint: In order to answer this question, we should know about the chemical formula of methyl cyanide and acetamide. By knowing about their chemical formula, we will come to know which functional groups are present and how they change into each other.
Complete Step By Step Answer:
Let’s understand this question in complete detail.
First, we should know what acetamide is. An acetamide is the simplest amide derived from acetic acids. It is as organic compound with the chemical formula $ C{H_3}CON{H_2} $
On the other hand, methyl cyanide is given. It is the simplest organic compound that consists of a nitrile group. It is the chemical compound with the chemical formula $ C{H_3}CN $ . It is often abbreviated as $ MeCN $ .
Now, here in the question, acetamide is converted into methyl cyanide which indicates that amide group i.e. $ - CON{H_2} $ is converted into cyanide group i.e. $ - CN $ .
Here is the following reaction that occurs:
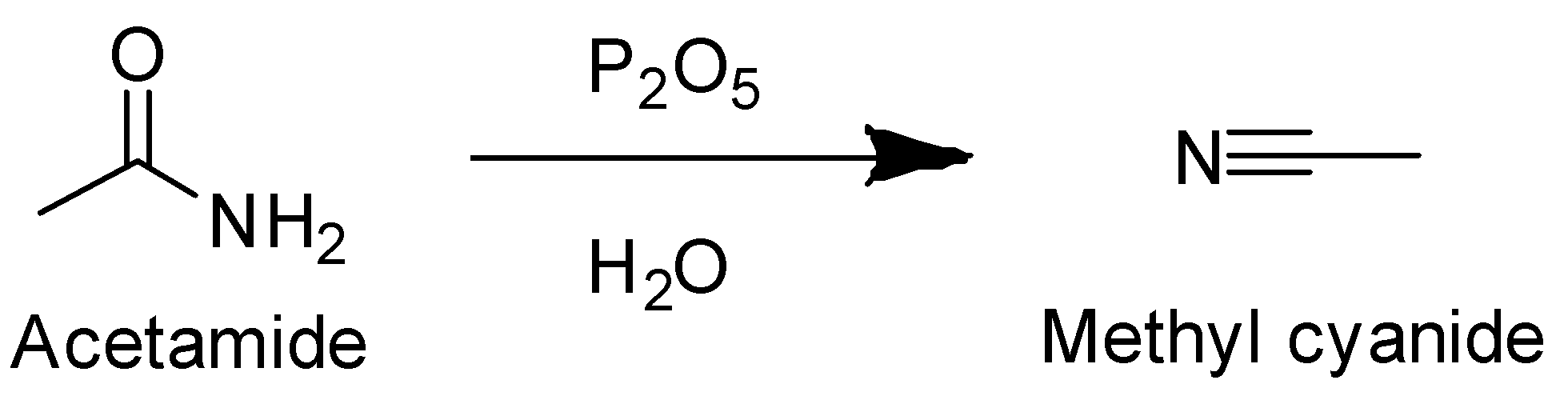
In the above reaction, when acetamide is heated with $ {P_2}{O_5} $ . A molecule of water is eliminated and methyl cyanide is obtained.
Note:
We must remember the chemical formula of the compound given and the reagents so that the conversion of functional groups takes place. For example- in the given reaction, acetamide contains the group i.e. $ - CON{H_2} $ which shows there is a carbon double bond with oxygen and a nitrogen is attached with that carbon. If we remove the oxygen and the two hydrogens attached with nitrogen then we will get a carbon triple bond with nitrogen i.e. a cyanide group. So, we need to choose a reagent which can remove water molecules.
Complete Step By Step Answer:
Let’s understand this question in complete detail.
First, we should know what acetamide is. An acetamide is the simplest amide derived from acetic acids. It is as organic compound with the chemical formula $ C{H_3}CON{H_2} $
On the other hand, methyl cyanide is given. It is the simplest organic compound that consists of a nitrile group. It is the chemical compound with the chemical formula $ C{H_3}CN $ . It is often abbreviated as $ MeCN $ .
Now, here in the question, acetamide is converted into methyl cyanide which indicates that amide group i.e. $ - CON{H_2} $ is converted into cyanide group i.e. $ - CN $ .
Here is the following reaction that occurs:

In the above reaction, when acetamide is heated with $ {P_2}{O_5} $ . A molecule of water is eliminated and methyl cyanide is obtained.
Note:
We must remember the chemical formula of the compound given and the reagents so that the conversion of functional groups takes place. For example- in the given reaction, acetamide contains the group i.e. $ - CON{H_2} $ which shows there is a carbon double bond with oxygen and a nitrogen is attached with that carbon. If we remove the oxygen and the two hydrogens attached with nitrogen then we will get a carbon triple bond with nitrogen i.e. a cyanide group. So, we need to choose a reagent which can remove water molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




