
What is meant by plasmolysis? How is it practically useful to us?
Answer
588.6k+ views
Hint: Plasmolysis is one of the results of osmosis. It happens in extreme conditions like when the cell is placed in a hypertonic solution.
Complete answer:
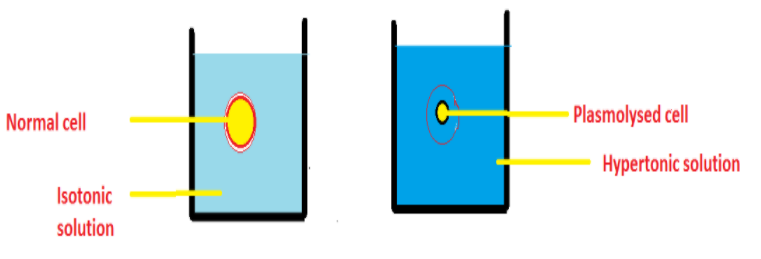
Plasmolysis can be defined as the shrinkage of the protoplasm when the cell is placed in a hypertonic solution. Hypertonic solution means a solution in which the solute concentration is greater than the solvent concentration. Example is sugar or salt solution. When a cell is placed in a hypertonic solution, the solvent concentration is higher inside the cell than its surrounding. The water molecules move out of the cell by the process of exosmosis. The turgor pressure decreases. As a result of which the cells shrink. Plasmolysis occurs in extreme conditions and occurs rarely in nature.
An example of plasmolysis is higher concentration of salt and sugar is used in pickles and Jam. Due to the higher concentration of sugar and salt, the microbes get plasmolysed. Thus jams and pickles can be stored for a longer time.
Additional Information: The outward movement of the solvent molecules from cell to surrounding is exosmosis. The inward movement of solvent from surrounding to cell is called endosmosis. An example of endosmosis is resins swell when soaked in water.
Note: The cells after plasmolysis shrink, whereas if the cells swell on deplasmolysis. For plasmolysis to take place, a hypertonic medium is necessary. The solvent particles move until the system becomes isotonic (same concentration of solute and solvent).
Complete answer:
Plasmolysis can be defined as the shrinkage of the protoplasm when the cell is placed in a hypertonic solution. Hypertonic solution means a solution in which the solute concentration is greater than the solvent concentration. Example is sugar or salt solution. When a cell is placed in a hypertonic solution, the solvent concentration is higher inside the cell than its surrounding. The water molecules move out of the cell by the process of exosmosis. The turgor pressure decreases. As a result of which the cells shrink. Plasmolysis occurs in extreme conditions and occurs rarely in nature.

An example of plasmolysis is higher concentration of salt and sugar is used in pickles and Jam. Due to the higher concentration of sugar and salt, the microbes get plasmolysed. Thus jams and pickles can be stored for a longer time.
Additional Information: The outward movement of the solvent molecules from cell to surrounding is exosmosis. The inward movement of solvent from surrounding to cell is called endosmosis. An example of endosmosis is resins swell when soaked in water.
Note: The cells after plasmolysis shrink, whereas if the cells swell on deplasmolysis. For plasmolysis to take place, a hypertonic medium is necessary. The solvent particles move until the system becomes isotonic (same concentration of solute and solvent).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




