
What is the maximum number of stereoisomers possible for discodermolide?
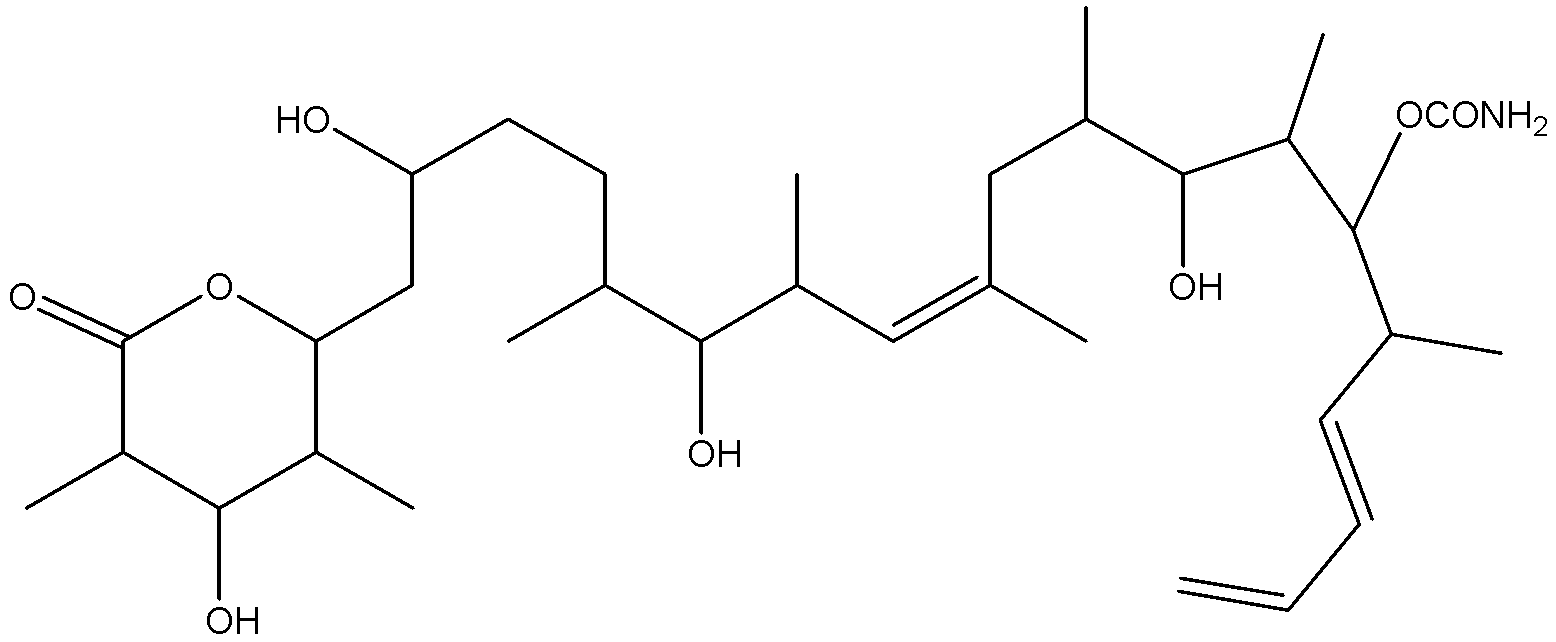
a) ${2^{14}}$
b) ${2^{15}}$
c) ${2^{16}}$
d) ${2^{17}}$

Answer
571.8k+ views
Hint: We know that Stereoisomers are the isomers that have the same molecular formula but differ in spatial arrangement of atoms. Stereoisomers can be subdivided into two groups,
-Optical isomers
-Geometrical isomers
Complete step by step solution:
We have to remember that an atom is stereogenic if exchanging any two iotas or gatherings of particles that are bound to it brings about a couple of stereoisomers. Up until this point, particles with no or only one stereogenic iota have been talked about. Frequently the circumstance is more intricate; without a doubt, there can be a few stereogenic particles in an atom.
As we know that a particle with only one stereogenic molecule has just two stereoisomers—the R and S enantiomers. In the event that there are two stereogenic particles in an atom, both can be either R or S. In this way, there are four prospects: RR, SS, RS, and SR.
Three stereogenic particles would prompt eight prospects: RRR, RRS, RSR, SRR, SSR, SRS, RSS, and SSS. The recipe for finding the most extreme number of stereoisomers X will be \[X = {2^n}\] where n is the quantity of stereogenic iotas in the particle.
Discodermolide has 13 chiral carbons and two geometrical centres which means the number of stereoisomers for discodermolide ${2^{15}}$.
Therefore, the option B is correct.
Note: If there is restricted rotation in a molecule there arises geometric isomerism. Geometric isomers are also known as Cis- trans isomerism.
-If the two atoms locked in same side of the molecule then it is called as cis isomers.
-If the two atoms are locked on opposite sides of the molecule then it is called trans isomers.
-Optical isomers
-Geometrical isomers
Complete step by step solution:
We have to remember that an atom is stereogenic if exchanging any two iotas or gatherings of particles that are bound to it brings about a couple of stereoisomers. Up until this point, particles with no or only one stereogenic iota have been talked about. Frequently the circumstance is more intricate; without a doubt, there can be a few stereogenic particles in an atom.
As we know that a particle with only one stereogenic molecule has just two stereoisomers—the R and S enantiomers. In the event that there are two stereogenic particles in an atom, both can be either R or S. In this way, there are four prospects: RR, SS, RS, and SR.
Three stereogenic particles would prompt eight prospects: RRR, RRS, RSR, SRR, SSR, SRS, RSS, and SSS. The recipe for finding the most extreme number of stereoisomers X will be \[X = {2^n}\] where n is the quantity of stereogenic iotas in the particle.
Discodermolide has 13 chiral carbons and two geometrical centres which means the number of stereoisomers for discodermolide ${2^{15}}$.
Therefore, the option B is correct.
Note: If there is restricted rotation in a molecule there arises geometric isomerism. Geometric isomers are also known as Cis- trans isomerism.
-If the two atoms locked in same side of the molecule then it is called as cis isomers.
-If the two atoms are locked on opposite sides of the molecule then it is called trans isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




