
Maximum enol content is in:
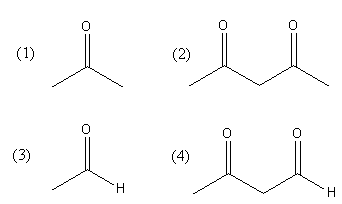

Answer
582.3k+ views
Hint: In keto form alpha-hydrogen shifts to oxygen forming a hydroxyl group. The newly formed structure is known as enol. The amount of enol in keto-enol form, depends upon how stable the enol form is.
Step by step answer: The functional group having double-bonded oxygen atom and two bonds with hydrogen or alkyl group is known as the keto group and the group having hydroxyl group attached with double-bonded carbon atom is known as the enol form.
The conversion of keto to enol is known as ketone-enol tautomerism.
The shifting of alpha-hydrogen of keto to double-bonded oxygen gives enol form.
The conversion of keto to enol of each compound is shown as follows:
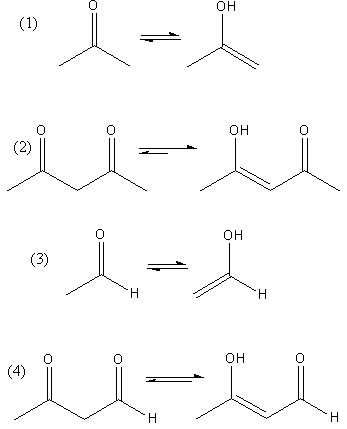
All the compound shows keto-enol tautomerism but the enol form of compound $(2)$ and $(4)$ is more stable due to hydrogen bonding.
The hydrogen bonding in compound $(2)$ and $(4)$ is shown as follows:
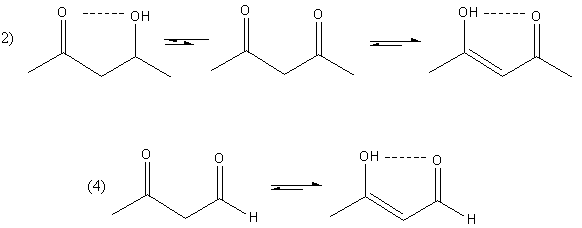
So, compounds $(2)$ and $(4)$ both enol form are stable due to the hydrogen bonding but in compound $(2)$, more amount of enol is present because, in compound $(2)$, two keto group is present whereas, in compound $(4)$, only one keto group is present.
So, the compound $(2)$ has maximum enol content.
So, options \[\left( 1 \right),{\text{ }}\left( 3 \right)\] and $(4)$ are not the correct option because all have enol form but in less amount due to less stability.
Therefore, option $(2)$ is correct.
Note: The keto form is more stable than the enol form because the carbon-oxygen double bond is more stable than the carbon-carbon double bond. If enol is present in more amounts than keto form, then there will be some additional stability factor in enol form such as hydrogen bonding.
Step by step answer: The functional group having double-bonded oxygen atom and two bonds with hydrogen or alkyl group is known as the keto group and the group having hydroxyl group attached with double-bonded carbon atom is known as the enol form.
The conversion of keto to enol is known as ketone-enol tautomerism.
The shifting of alpha-hydrogen of keto to double-bonded oxygen gives enol form.
The conversion of keto to enol of each compound is shown as follows:

All the compound shows keto-enol tautomerism but the enol form of compound $(2)$ and $(4)$ is more stable due to hydrogen bonding.
The hydrogen bonding in compound $(2)$ and $(4)$ is shown as follows:

So, compounds $(2)$ and $(4)$ both enol form are stable due to the hydrogen bonding but in compound $(2)$, more amount of enol is present because, in compound $(2)$, two keto group is present whereas, in compound $(4)$, only one keto group is present.
So, the compound $(2)$ has maximum enol content.
So, options \[\left( 1 \right),{\text{ }}\left( 3 \right)\] and $(4)$ are not the correct option because all have enol form but in less amount due to less stability.
Therefore, option $(2)$ is correct.
Note: The keto form is more stable than the enol form because the carbon-oxygen double bond is more stable than the carbon-carbon double bond. If enol is present in more amounts than keto form, then there will be some additional stability factor in enol form such as hydrogen bonding.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




