
What is the mass of three moles of \[{C_2}{H_2}\]?
Answer
521.4k+ views
Hint: We have to remember that the acetylene is a simplest alkyne and it is a hydrocarbon having the formula \[{C_2}{H_2}\]. And this compound is also known as ethyne. Acetylene is a colorless gas and it is used as fuel.
Complete answer:
We also know that a mole is used to specify the amount of material and it contains \[6.022 \times {10^{23}}\] particles. Hence, one mole is equal to \[6.022 \times {10^{23}}\] particles and this number is called Avogadro number. The mole is mainly used to express the number of products and the reactants. Hence, it is a count of particles. The mass of one mole of substance is equal to the molar mass of the compound. Therefore, the number of moles is equal to the amount of samples. Acetylene is a simplest alkyne and it is a hydrocarbon having the formula \[{C_2}{H_2}\]. We must remember that acetylene is a compound with two carbon atoms and two hydrogen atoms. The mass of three mole of acetylene is equal to its molecular mass.
The molar mass of carbon, C\[ = 12\]
The molar mass of hydrogen, H\[ = 1\]
To find the mass of acetylene, add the molar mass of elements. Therefore,
The mass of three mole of acetylene \[ = 2 \times 12 + 2 \times 1 = 26\]
Hence the mass of three mole of acetylene is equal to \[26g\].
The structure of acetylene can be drawn as,
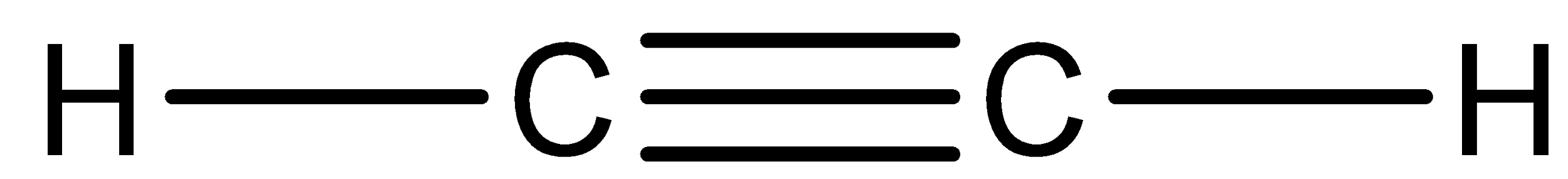
And this acetylene is mainly used in the fabrication industry and it is used as fuel for welding. And it is a highly unstable compound. It may lead to explosion under high pressure or high temperature conditions.
Note:
We must have to remember that acetylene is the simplest alkyne having the formula, \[{C_2}{H_2}\]. And it has two carbon atoms and two hydrogen atoms. The mass of a compound is equal to its molecular mass. Hence, the mass of three mole of acetylene is equal to \[26\]g.
Complete answer:
We also know that a mole is used to specify the amount of material and it contains \[6.022 \times {10^{23}}\] particles. Hence, one mole is equal to \[6.022 \times {10^{23}}\] particles and this number is called Avogadro number. The mole is mainly used to express the number of products and the reactants. Hence, it is a count of particles. The mass of one mole of substance is equal to the molar mass of the compound. Therefore, the number of moles is equal to the amount of samples. Acetylene is a simplest alkyne and it is a hydrocarbon having the formula \[{C_2}{H_2}\]. We must remember that acetylene is a compound with two carbon atoms and two hydrogen atoms. The mass of three mole of acetylene is equal to its molecular mass.
The molar mass of carbon, C\[ = 12\]
The molar mass of hydrogen, H\[ = 1\]
To find the mass of acetylene, add the molar mass of elements. Therefore,
The mass of three mole of acetylene \[ = 2 \times 12 + 2 \times 1 = 26\]
Hence the mass of three mole of acetylene is equal to \[26g\].
The structure of acetylene can be drawn as,
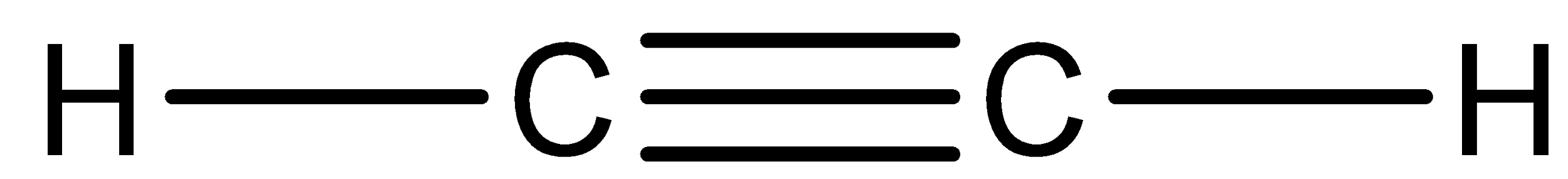
And this acetylene is mainly used in the fabrication industry and it is used as fuel for welding. And it is a highly unstable compound. It may lead to explosion under high pressure or high temperature conditions.
Note:
We must have to remember that acetylene is the simplest alkyne having the formula, \[{C_2}{H_2}\]. And it has two carbon atoms and two hydrogen atoms. The mass of a compound is equal to its molecular mass. Hence, the mass of three mole of acetylene is equal to \[26\]g.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




