
What is Malachite green? How is it prepared?
Answer
587.7k+ views
Hint: Malachite green is a compound that is used as a dye in textile industries and it is also used in aquariums as an antibacterial, antifungal, and as antiparasitic. Malachite can be prepared by condensation of benzaldehyde and dimethylaniline.
Complete step by step answer:
Malachite green is an organic compound and it is the simplest triphenylmethane dye. It has a chemical formula ${C_{23}}{H_{25}}Cl{N_2}$. It appears as green. It is used as a green colored dye in the textile industry and traditionally used as a dye for materials like silk, leather, and paper. It is also known for its anti-fungal properties in aquaculture and thus use in aquariums as an antifungal, antibacterial and as antiparasitic. It has been used to control the fungus Saprolegnia, a water mold that kills the eggs and young fry.
We can prepare Malachite green by the condensation of benzaldehyde and dimethylaniline. When we react benzaldehyde and dimethylaniline in the presence of zinc chloride, it forms a leuco base (that is a colorless base) and then we oxidize this base with the excess hydrogen chloride to form the required malachite green compound. Here, benzaldehyde and dimethylaniline react with each other in the molecular ratio of $1:2$. This reaction can be shown as :
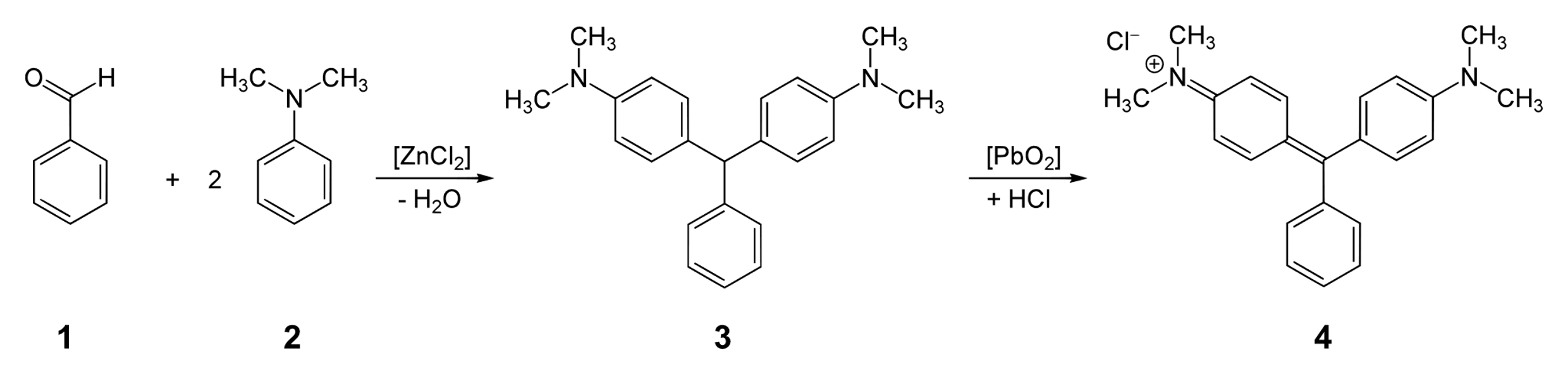
Note:
We need to remember that although the given compound is called malachite green, it is not prepared from the mineral malachite. This name (malachite green) just comes from the similarity of color.
Complete step by step answer:
Malachite green is an organic compound and it is the simplest triphenylmethane dye. It has a chemical formula ${C_{23}}{H_{25}}Cl{N_2}$. It appears as green. It is used as a green colored dye in the textile industry and traditionally used as a dye for materials like silk, leather, and paper. It is also known for its anti-fungal properties in aquaculture and thus use in aquariums as an antifungal, antibacterial and as antiparasitic. It has been used to control the fungus Saprolegnia, a water mold that kills the eggs and young fry.
We can prepare Malachite green by the condensation of benzaldehyde and dimethylaniline. When we react benzaldehyde and dimethylaniline in the presence of zinc chloride, it forms a leuco base (that is a colorless base) and then we oxidize this base with the excess hydrogen chloride to form the required malachite green compound. Here, benzaldehyde and dimethylaniline react with each other in the molecular ratio of $1:2$. This reaction can be shown as :

Note:
We need to remember that although the given compound is called malachite green, it is not prepared from the mineral malachite. This name (malachite green) just comes from the similarity of color.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




