
How can I make the atomic model of boron?
Answer
559.2k+ views
Hint As the atomic number of Boron is 5, so the atom will consist of 5 protons and 5 electrons. Using mass number as 11, the number of neutrons will be 6. Both the protons and neutrons will be in the centre of the atom, inside the nucleus and the electrons will be revolving in the orbits.
Complete step by step solution:
In order to answer the question, we need to learn about the structure of the atom. Now, each and every atom in the universe is made up of 3 things, namely proton, neutron and the electron. There is a small nucleus present in the centre of every atom and this neutron occupies the proton and neutron. Together, it is called the nucleon. The nucleus is very small and occupies a very small space compared to the atom. However, it is very much concentrated and almost immobile. Consider a circular cricket field and there is a ball in between the field. This ball represents the size of the nucleus. The proton is positively charged, the neutron is neutral and the electrons are negatively charged. Around the nucleus, there are orbits and the electrons revolve around these orbits in their respective shells. Electrons are the only mobile part of an atom.
Now, boron has an atomic number of 5. It means that boron has 5 protons and 5 electrons. Mass number of the boron is 11 which means that the number of neutrons is 11-5 = 6. So, let us construct the hypothetical model of the atom of boron:
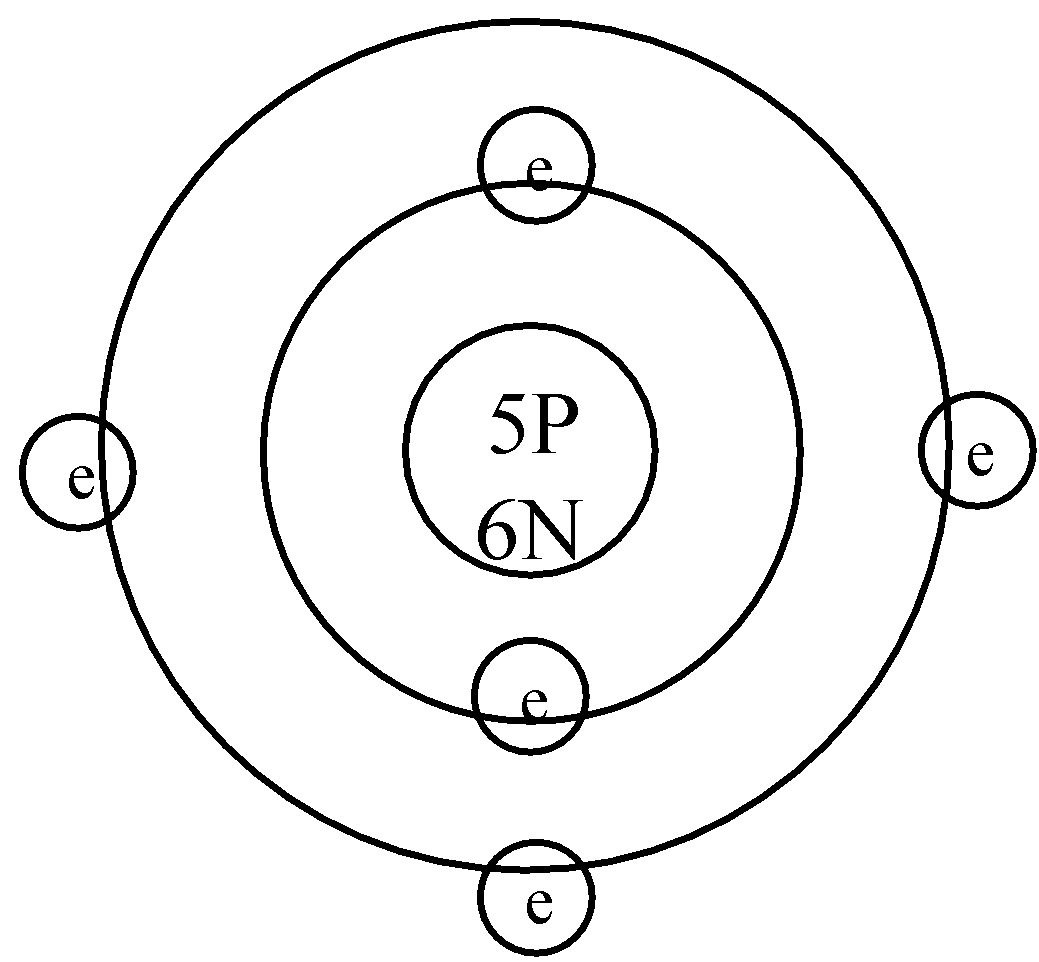
P stands for proton and N stands for neutron. Also, the maximum number of electrons in a shell is $2{{n}^{2}}$, where n is the shell number. As in the first shell, n=1, so a maximum of 2 electrons can fit in the first shell. A new shell is needed to store the rest electrons.
NOTE: As Boron has an atomic number of 5 it is said that it has both 5 protons and 5 neutrons. It is so because the overall charge of the boron atom is 0. Both the 5 protons and 5 neutrons give the net charge of the atom to be 0.
Complete step by step solution:
In order to answer the question, we need to learn about the structure of the atom. Now, each and every atom in the universe is made up of 3 things, namely proton, neutron and the electron. There is a small nucleus present in the centre of every atom and this neutron occupies the proton and neutron. Together, it is called the nucleon. The nucleus is very small and occupies a very small space compared to the atom. However, it is very much concentrated and almost immobile. Consider a circular cricket field and there is a ball in between the field. This ball represents the size of the nucleus. The proton is positively charged, the neutron is neutral and the electrons are negatively charged. Around the nucleus, there are orbits and the electrons revolve around these orbits in their respective shells. Electrons are the only mobile part of an atom.
Now, boron has an atomic number of 5. It means that boron has 5 protons and 5 electrons. Mass number of the boron is 11 which means that the number of neutrons is 11-5 = 6. So, let us construct the hypothetical model of the atom of boron:

P stands for proton and N stands for neutron. Also, the maximum number of electrons in a shell is $2{{n}^{2}}$, where n is the shell number. As in the first shell, n=1, so a maximum of 2 electrons can fit in the first shell. A new shell is needed to store the rest electrons.
NOTE: As Boron has an atomic number of 5 it is said that it has both 5 protons and 5 neutrons. It is so because the overall charge of the boron atom is 0. Both the 5 protons and 5 neutrons give the net charge of the atom to be 0.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




