
How will you make (obtain) from chloroform:
A. Chloretone
B. Phenyl isocyanide
C. Acetylene
D. Salicylaldehyde
Answer
557.1k+ views
Hint: Chloroform is an organic compound with chemical formula \[CHC{l_3}\]. It is composed of carbon, hydrogen and three chlorine atoms. It is used as a chlorinated solvent in organic synthesis.
Complete step by step answer:
The chloroform is a chlorinated organic compound. It is polar solvent and readily dissolves most of the organic compounds. It is also called trichloromethane or chlorinated solvent. It is colourless, volatile and dense liquid and has a characteristic strong smell.
Let us synthesize the given compounds using chloroform as a starting material.
A. Chloretone.
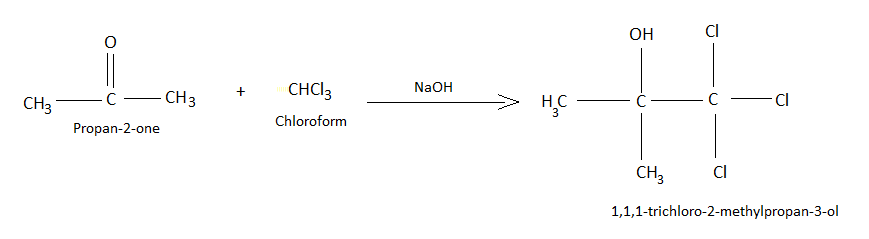
Chloretone is a chemical used as a preservative. The compound is also known as chlorobutanol, or chlorbutol. The IUPAC name is \[1,1,1\] -trichloro-\[2\]-methyl-\[2\]-propanol. It is prepared by reaction acetone with chloroform in presence of sodium hydroxide.
B. Phenyl isocyanide
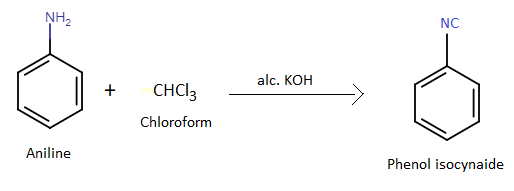
Phenyl isocyanide is an organic compound containing an isocyanate functional group. Phenyl isocyanide is prepared by treating phenyl amine or aniline with chloroform in presence of alcoholic potassium hydroxide. The reaction is named as carbylamine reaction. This reaction is used as a confirmatory test for the identification of aromatic amine. The generation of unpleasant smell indicates that an isocyanide has formed in the reaction.
C. Acetylene
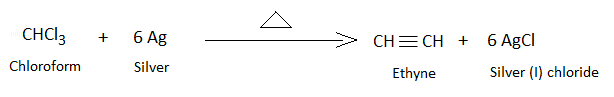
Acetylene is the primary ethyne hydrocarbon with chemical formula \[{C_2}{H_2}\]. It is formed by the reaction of chloroform and silver power under heating conditions. \[AgCl\]is formed as the side product.
D. Salicylaldehyde
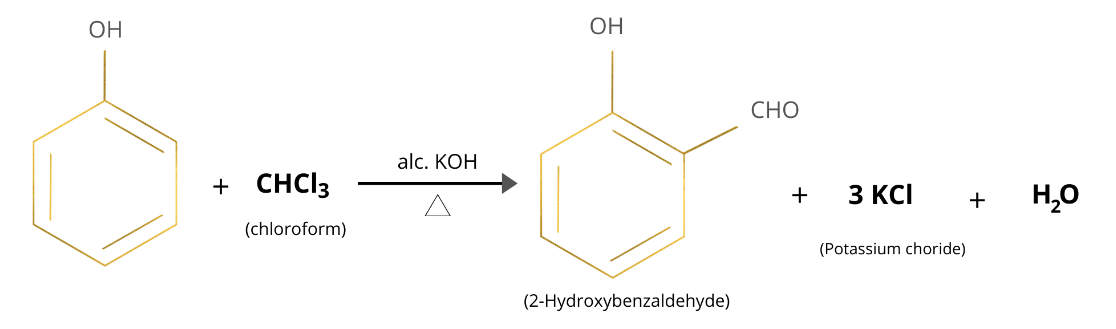
Salicylaldehyde is ortho-hydroxy benzaldehyde or \[2\]-hydroxy benzaldehyde. Salicylaldehyde is prepared using Reimer Tiemann reaction. It is synthesized by reaction phenol with chloroform in presence of alcohol \[KOH\]. An aldehyde group is introduced on the benzene ring ortho to the hydroxyl group.
Note:
The mechanism of these reactions include the formation of dichlorocarbene from chloroform by loss of hydrogen and a chlorine atom using basic conditions. The dichlorocarbene acts as an electrophile and attacks electron-rich species like alkene or benzene rings. It also acts as a nucleophile and attacks electron deficient carbonyl carbon.
Complete step by step answer:
The chloroform is a chlorinated organic compound. It is polar solvent and readily dissolves most of the organic compounds. It is also called trichloromethane or chlorinated solvent. It is colourless, volatile and dense liquid and has a characteristic strong smell.
Let us synthesize the given compounds using chloroform as a starting material.
A. Chloretone.
Chloretone is a chemical used as a preservative. The compound is also known as chlorobutanol, or chlorbutol. The IUPAC name is \[1,1,1\] -trichloro-\[2\]-methyl-\[2\]-propanol. It is prepared by reaction acetone with chloroform in presence of sodium hydroxide.

B. Phenyl isocyanide
Phenyl isocyanide is an organic compound containing an isocyanate functional group. Phenyl isocyanide is prepared by treating phenyl amine or aniline with chloroform in presence of alcoholic potassium hydroxide. The reaction is named as carbylamine reaction. This reaction is used as a confirmatory test for the identification of aromatic amine. The generation of unpleasant smell indicates that an isocyanide has formed in the reaction.

C. Acetylene
Acetylene is the primary ethyne hydrocarbon with chemical formula \[{C_2}{H_2}\]. It is formed by the reaction of chloroform and silver power under heating conditions. \[AgCl\]is formed as the side product.

D. Salicylaldehyde
Salicylaldehyde is ortho-hydroxy benzaldehyde or \[2\]-hydroxy benzaldehyde. Salicylaldehyde is prepared using Reimer Tiemann reaction. It is synthesized by reaction phenol with chloroform in presence of alcohol \[KOH\]. An aldehyde group is introduced on the benzene ring ortho to the hydroxyl group.

Note:
The mechanism of these reactions include the formation of dichlorocarbene from chloroform by loss of hydrogen and a chlorine atom using basic conditions. The dichlorocarbene acts as an electrophile and attacks electron-rich species like alkene or benzene rings. It also acts as a nucleophile and attacks electron deficient carbonyl carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




