
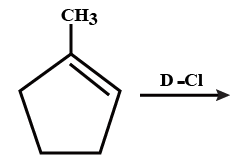
What is the major product expected from the following reaction? Where D is an isotope of hydrogen.
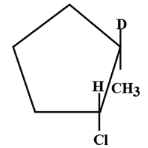
A.

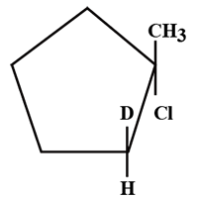
B.

C.

D.





Answer
584.7k+ views
Hint: We can understand that this is an electrophilic addition reaction for an unsymmetrical alkene. So we can apply the markovnikov rule to find the product of the reaction and the position of D in the final product.
Complete step by step solution:
We can see the following reaction is an example of electrophilic addition reaction. So, before discussing that let’s first discuss the types of alkene. There are usually two types of alkene, Symmetrical and unsymmetrical alkenes. In symmetrical alkene, the carbon atom across the double bond has the same number of carbon atoms. For example \[{H_2}C = C{H_2}\]. Unsymmetrical alkenes are the ones in which the carbon atom surrounding the double bond has different numbers of hydrogen atoms. For example, .
The alkene given to us is an unsymmetrical alkene and the additional reaction in unsymmetrical alkene takes place according to markovnikov rule. According to this when an unsymmetrical alkene is treated with unsymmetrical reagent like \[\;HBr\],\[\;HCl\] and etc. the negative part that is the anion of the reagent goes to the carbon atom across the double bond having lesser number of hydrogen atom.
In our question the anion part of reagent is \[C{l^ - }\]. So it will go to the carbon atom which having the branch of \[C{H_3}\], whereas the \[{D^ + }\] which is an isotope of hydrogen goes to the carbon atom across the double bond having higher number of hydrogen. So, the answer will be
So, we can conclude that the answer to this question is option A.
Note: We should remember that what are the symmetrical and unsymmetrical alkenes along with the rules they follow for additional reaction. Such questions are frequently asked in many exams, so we must practice them diligently.
And we must know that Markovnikov rule is an empirical rule and we can use it to predict region selectivity of electrophilic addition reactions of alkenes and alkynes.
Complete step by step solution:
We can see the following reaction is an example of electrophilic addition reaction. So, before discussing that let’s first discuss the types of alkene. There are usually two types of alkene, Symmetrical and unsymmetrical alkenes. In symmetrical alkene, the carbon atom across the double bond has the same number of carbon atoms. For example \[{H_2}C = C{H_2}\]. Unsymmetrical alkenes are the ones in which the carbon atom surrounding the double bond has different numbers of hydrogen atoms. For example, .
The alkene given to us is an unsymmetrical alkene and the additional reaction in unsymmetrical alkene takes place according to markovnikov rule. According to this when an unsymmetrical alkene is treated with unsymmetrical reagent like \[\;HBr\],\[\;HCl\] and etc. the negative part that is the anion of the reagent goes to the carbon atom across the double bond having lesser number of hydrogen atom.
In our question the anion part of reagent is \[C{l^ - }\]. So it will go to the carbon atom which having the branch of \[C{H_3}\], whereas the \[{D^ + }\] which is an isotope of hydrogen goes to the carbon atom across the double bond having higher number of hydrogen. So, the answer will be

So, we can conclude that the answer to this question is option A.
Note: We should remember that what are the symmetrical and unsymmetrical alkenes along with the rules they follow for additional reaction. Such questions are frequently asked in many exams, so we must practice them diligently.
And we must know that Markovnikov rule is an empirical rule and we can use it to predict region selectivity of electrophilic addition reactions of alkenes and alkynes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




