
Major product ‘B’ of the given reaction is:
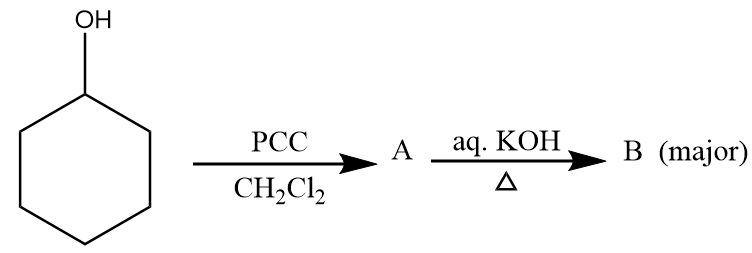
A.
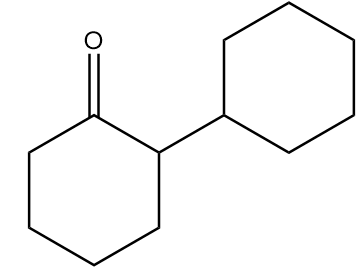
B.
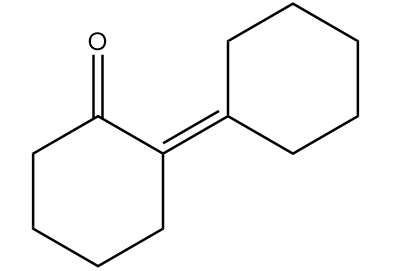
C.
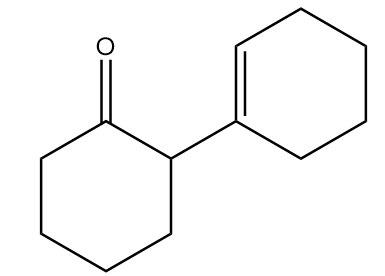
D.





Answer
510.6k+ views
Hint: Aldol condensation: It is a reaction in organic chemistry in which aldehyde and ketones which have alpha hydrogens reacts with dilute alkali solution to form $ \beta - $ hydroxy aldehydes which are termed as aldol or $ \beta - $ hydroxy ketones which are termed as ketols and on further heating, formation of $ \alpha - \beta $ unsaturated aldehyde or ketone will take place respectively.
Complete answer:
The mechanism for the given reaction sequence is as follows:
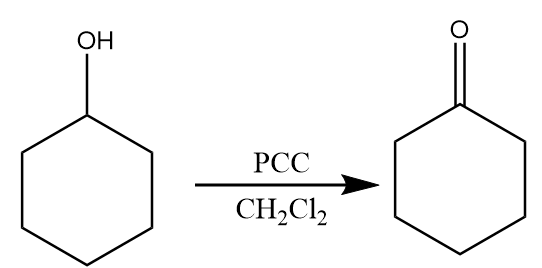
Step-1: PCC is a mild oxidizing agent and oxidizes the alcohols into aldehyde (in case of primary alcohol) or ketones (in case of secondary alcohol) in the presence of dichloromethane. The reaction proceeds as follows:
Step-2: The ketone formed in step-1 will undergo aldol condensation in the presence of base using following reaction mechanism:
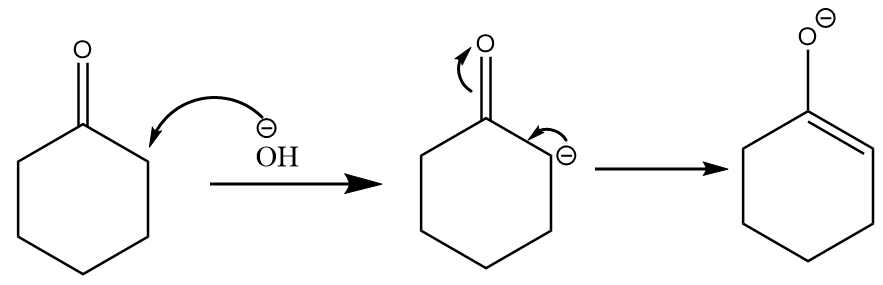
(a) The hydroxide ion present in the medium will extract alpha hydrogen from the ketone to form an enolate. The reaction proceeds as follows:
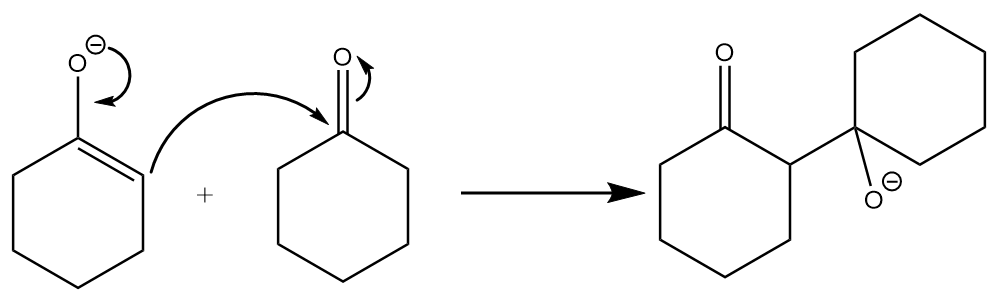
(b) The enolate will react with another mole of ketone and the attack will take place at the carbonyl centre and an intermediate will be formed. The reaction proceeds as follows:
(c) On acidic workup of the intermediate formed in the previous step, then aldol i.e., beta-hydroxy ketone will be formed. The reaction proceeds as follows:
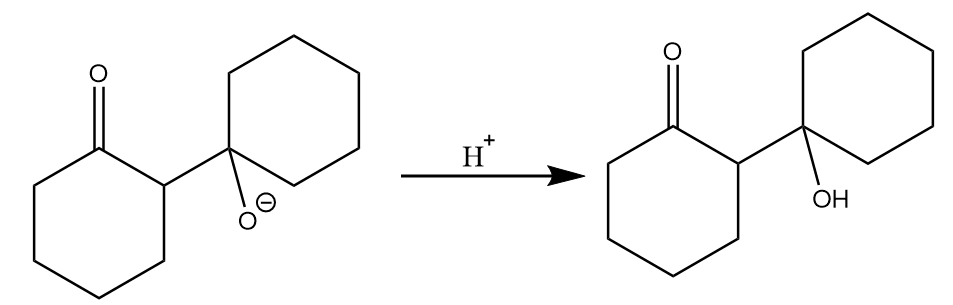
(d) On heating dehydration of aldol takes place by the removal of water molecules and the final product is formed. The reaction proceeds as follows:
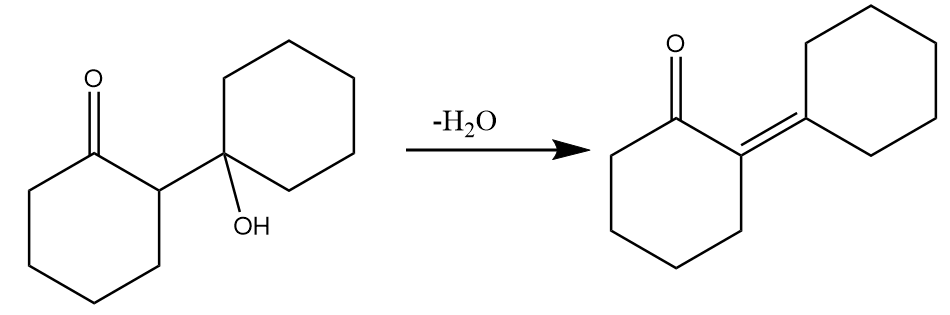
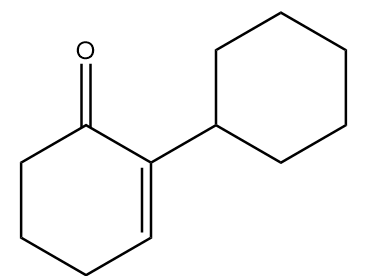
Hence, the major product ‘B’ formed in the given reaction sequence is as follows:
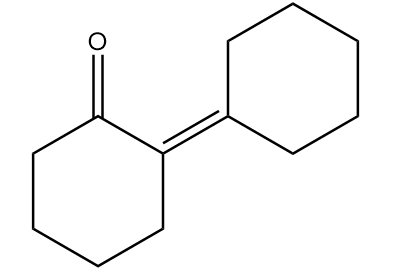
Therefore, option (B) is the correct answer.
Note:
It is important to note that for a compound to undergo aldol condensation, it is mandatory for that compound to have alpha hydrogens i.e., hydrogen atoms bonded to the carbon atom adjacent to the carbonyl group. If there is no alpha hydrogen present within the compound, then the Cannizzaro reaction will take place under the same basic conditions.
Complete answer:
The mechanism for the given reaction sequence is as follows:
Step-1: PCC is a mild oxidizing agent and oxidizes the alcohols into aldehyde (in case of primary alcohol) or ketones (in case of secondary alcohol) in the presence of dichloromethane. The reaction proceeds as follows:

Step-2: The ketone formed in step-1 will undergo aldol condensation in the presence of base using following reaction mechanism:
(a) The hydroxide ion present in the medium will extract alpha hydrogen from the ketone to form an enolate. The reaction proceeds as follows:

(b) The enolate will react with another mole of ketone and the attack will take place at the carbonyl centre and an intermediate will be formed. The reaction proceeds as follows:

(c) On acidic workup of the intermediate formed in the previous step, then aldol i.e., beta-hydroxy ketone will be formed. The reaction proceeds as follows:

(d) On heating dehydration of aldol takes place by the removal of water molecules and the final product is formed. The reaction proceeds as follows:

Hence, the major product ‘B’ formed in the given reaction sequence is as follows:

Therefore, option (B) is the correct answer.
Note:
It is important to note that for a compound to undergo aldol condensation, it is mandatory for that compound to have alpha hydrogens i.e., hydrogen atoms bonded to the carbon atom adjacent to the carbonyl group. If there is no alpha hydrogen present within the compound, then the Cannizzaro reaction will take place under the same basic conditions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




