
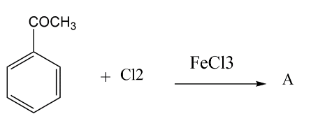
Major product “A” of the reaction is?
A.

B.
C.
D.





Answer
547.2k+ views
Hint: The reaction is about the ortho -para directing nature of acetyl group. We have an acetyl group on a benzene ring and it is having a reaction with chlorine in presence of \[FeC{l_3}\] . Thus we get that substitution will happen at different positions of benzene. So, if you know the nature of the attacking group which is on the benzene ring we can easily make a product for the reaction.
Complete step-by-step answer:Let’s start with the reagent which is given for the reaction, it is chlorine in the presence of ferric chloride \[FeC{l_3}\]. So our first step is to see the nature of groups on benzene rings. There are two types of directing groups. There are ortho- para directing groups which give substitution products at ortho and para site. While on the other hand we have a Meta directing group, which gives substitution at meta position.
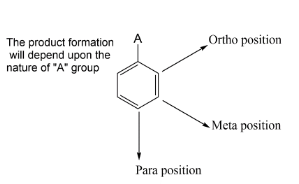
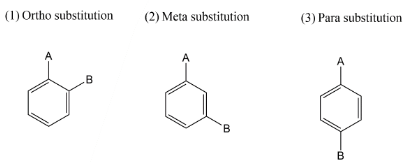
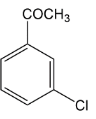
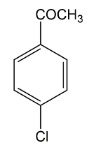
In the question we have given acetyl group $( - COC{H_3})\,group$ which is ortho and para directing so chlorine must be substituted at any one position among ortho position and para position. It was found that among ortho-para products, para products are always found as major. Thus in this reaction also we get para as a major product. Let’s see the mechanism of the above reaction.
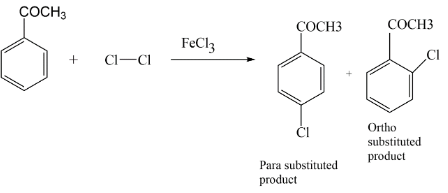
Thus according to the options, the correct answer will be option C where we are getting a para-substituted product.
Note: For each type of reaction whenever we need to do substitution, we need to check the directing nature of the group. There are many group which are ortho and para directing but are not activating like $( - COOH, - N{O_2}etc)$ here it means they decrease the reacting tendency of benzene while many groups are activating groups like $( - OH, - N{H_2}etc)$ which activates the benzene ring for reaction.
Complete step-by-step answer:Let’s start with the reagent which is given for the reaction, it is chlorine in the presence of ferric chloride \[FeC{l_3}\]. So our first step is to see the nature of groups on benzene rings. There are two types of directing groups. There are ortho- para directing groups which give substitution products at ortho and para site. While on the other hand we have a Meta directing group, which gives substitution at meta position.


In the question we have given acetyl group $( - COC{H_3})\,group$ which is ortho and para directing so chlorine must be substituted at any one position among ortho position and para position. It was found that among ortho-para products, para products are always found as major. Thus in this reaction also we get para as a major product. Let’s see the mechanism of the above reaction.

Thus according to the options, the correct answer will be option C where we are getting a para-substituted product.
Note: For each type of reaction whenever we need to do substitution, we need to check the directing nature of the group. There are many group which are ortho and para directing but are not activating like $( - COOH, - N{O_2}etc)$ here it means they decrease the reacting tendency of benzene while many groups are activating groups like $( - OH, - N{H_2}etc)$ which activates the benzene ring for reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




