
What is the major organic compound that will be formed in the following reaction?
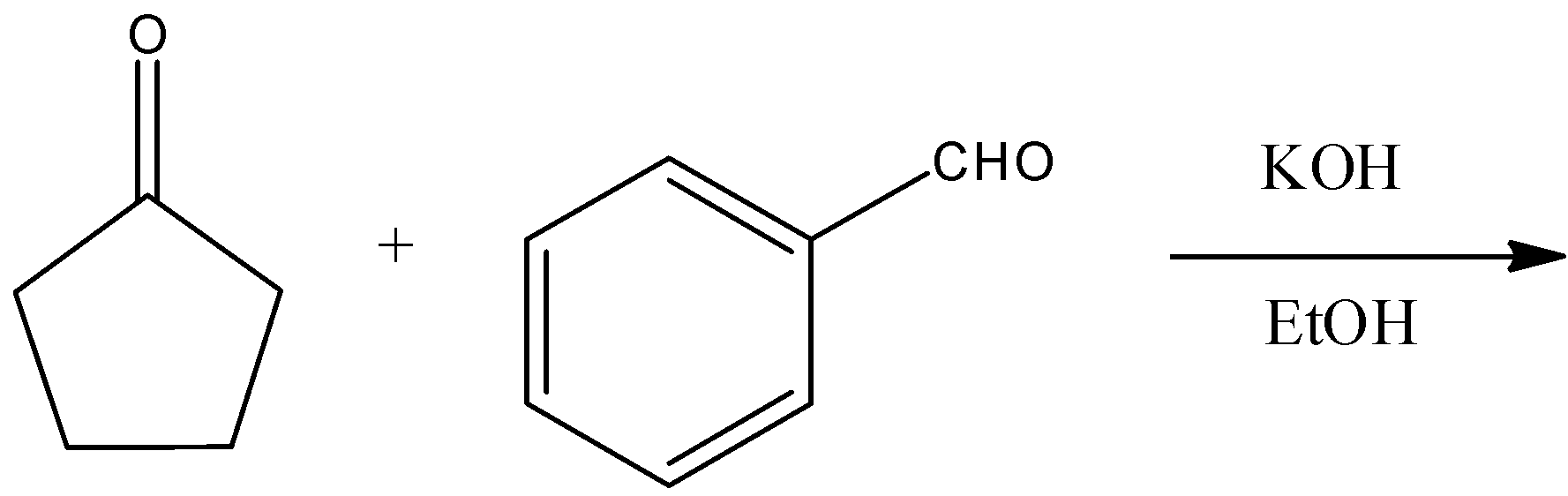
(A)
(B)
(C)
(D)
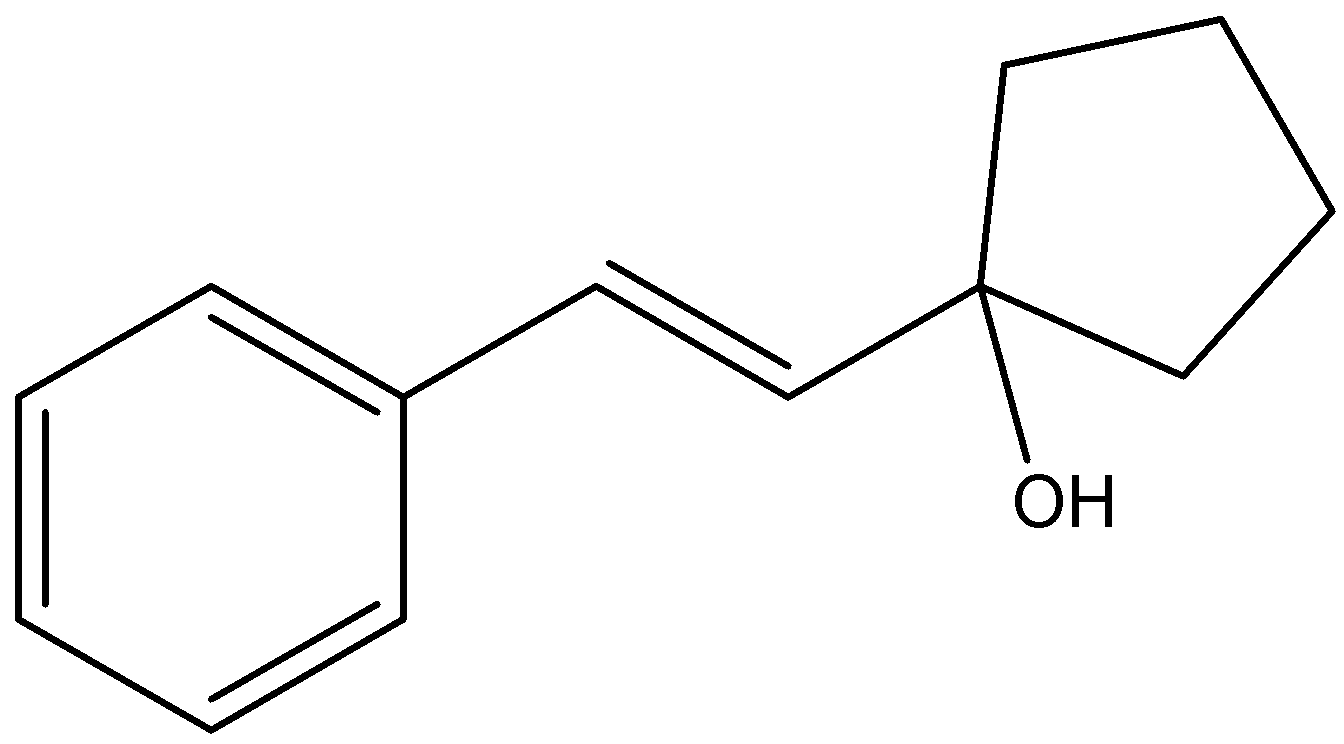





Answer
578.1k+ views
Hint: In the presence of strong bases like potassium hydroxide, the organic compounds which have acidic hydrogen or active hydrogen may lose hydrogen atoms and form carbanion. The hydrogen atoms that are $\alpha $ to the carbonyl group are acidic hydrogen atoms.
Complete answer:
We will try to understand the nature of reagents and find the answer.
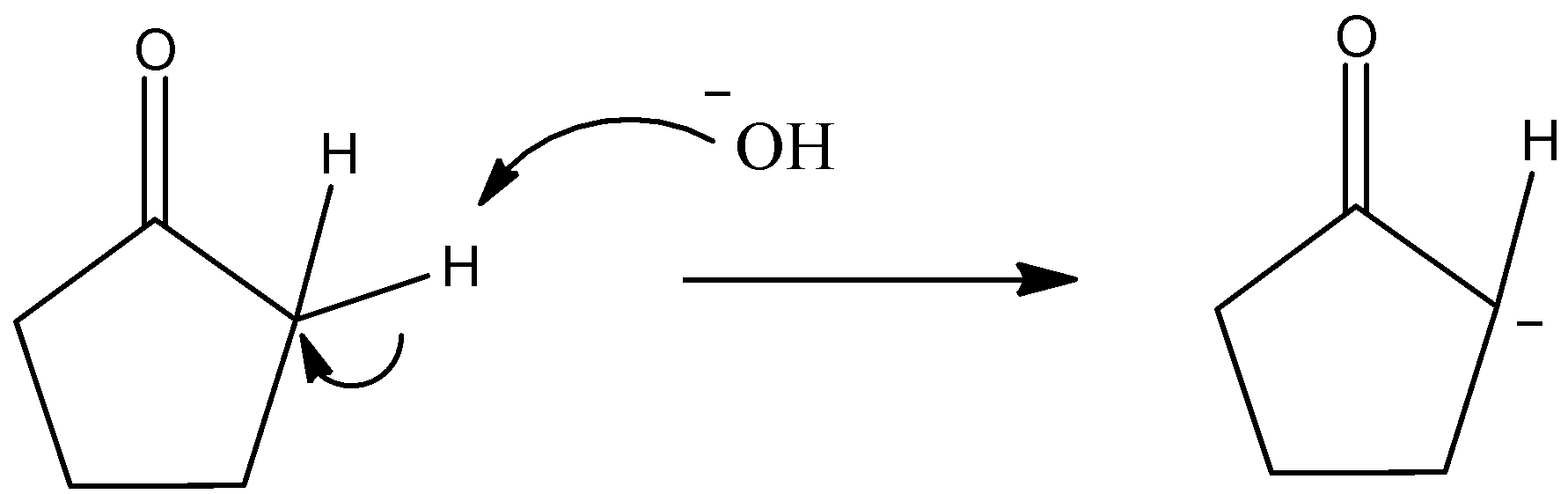
- Potassium hydroxide (KOH) is a strong base. As it is a strong base, it can accept acidic protons if available.
- We know that the $\alpha $-hydrogen of a ketone group is acidic because that C-H bond is more polar and hence the hydrogen atom becomes acidic in nature.
- So, KOH will absorb that hydrogen atom from cyclopentanone and will form a carbanion. The $H{O^ - }$ ion will attack the acidic proton of cyclopentanone to give an anionic intermediate.
- This carbanion now has a negatively charged carbon atom and it is highly nucleophilic. So, it will attack the electrophilic carbonyl carbon atom of benzaldehyde. So, it will form an alcohol. This alcohol is not much stable and then water will be lost to give alkene as a final product. The mechanism can be given as under.
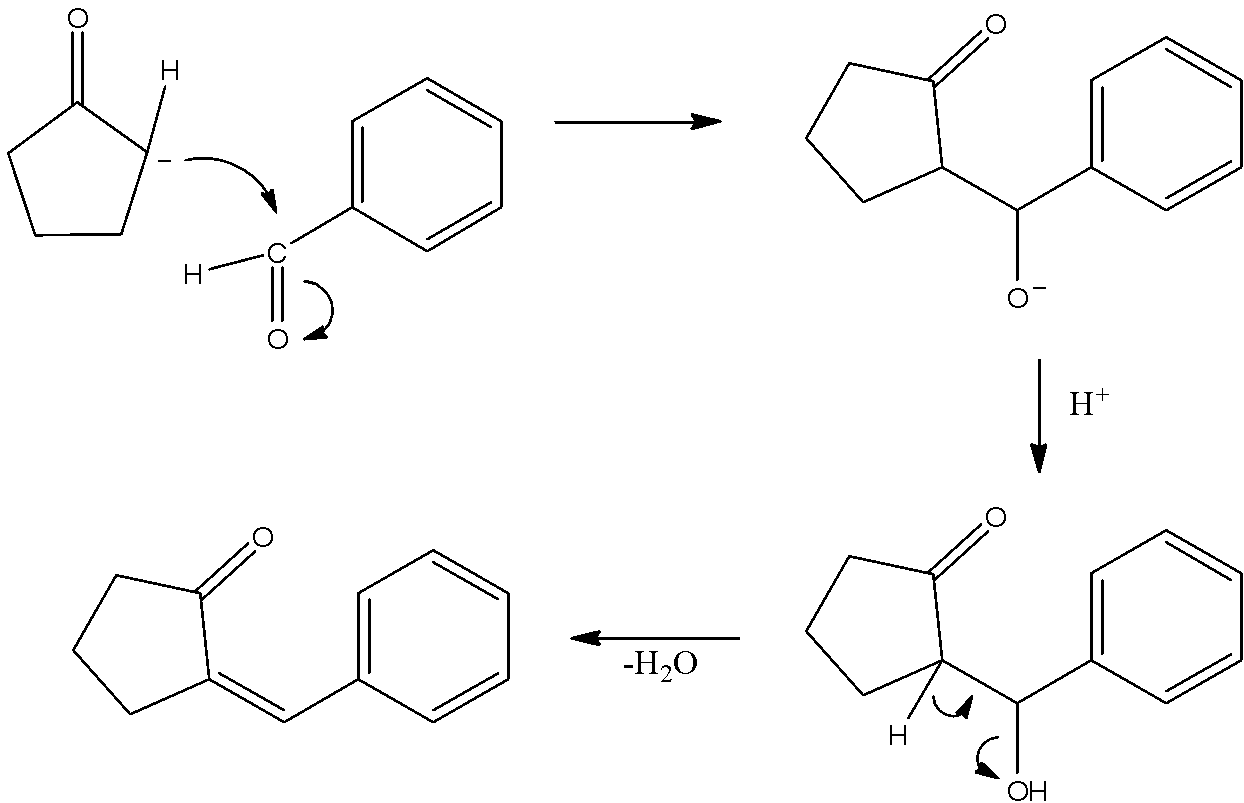
- Thus, we will obtain a compound which also has a carbon-carbon double bond.
Therefore, the correct answer of the question is (A).
Note:
Note that electrophilic substitution reaction will not occur as only electrophilic atom in cyclopentanone molecule is carbonyl carbon and it is also not electrophilic enough to give electrophilic substitution reaction. Alkene formation will be favored here because the resultant alkene will be conjugated with benzene and the ketone group. So, those alkenes are more stable and their formation is more favorable.
Complete answer:
We will try to understand the nature of reagents and find the answer.
- Potassium hydroxide (KOH) is a strong base. As it is a strong base, it can accept acidic protons if available.
- We know that the $\alpha $-hydrogen of a ketone group is acidic because that C-H bond is more polar and hence the hydrogen atom becomes acidic in nature.
- So, KOH will absorb that hydrogen atom from cyclopentanone and will form a carbanion. The $H{O^ - }$ ion will attack the acidic proton of cyclopentanone to give an anionic intermediate.

- This carbanion now has a negatively charged carbon atom and it is highly nucleophilic. So, it will attack the electrophilic carbonyl carbon atom of benzaldehyde. So, it will form an alcohol. This alcohol is not much stable and then water will be lost to give alkene as a final product. The mechanism can be given as under.

- Thus, we will obtain a compound which also has a carbon-carbon double bond.
Therefore, the correct answer of the question is (A).
Note:
Note that electrophilic substitution reaction will not occur as only electrophilic atom in cyclopentanone molecule is carbonyl carbon and it is also not electrophilic enough to give electrophilic substitution reaction. Alkene formation will be favored here because the resultant alkene will be conjugated with benzene and the ketone group. So, those alkenes are more stable and their formation is more favorable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




