
: m- nitro benzoic acid can be obtained by:
A.(i),(ii)
B.(ii),(iii)
C.(i),(ii),(iii)
D.(i),(iii)

Answer
565.5k+ views
Hint: To answer this question, you must recall the nature of the functional group present on the benzene ring when it is being nitrated. The functional group present must be a meta- directing group in order to obtain the required product.
Complete answer:
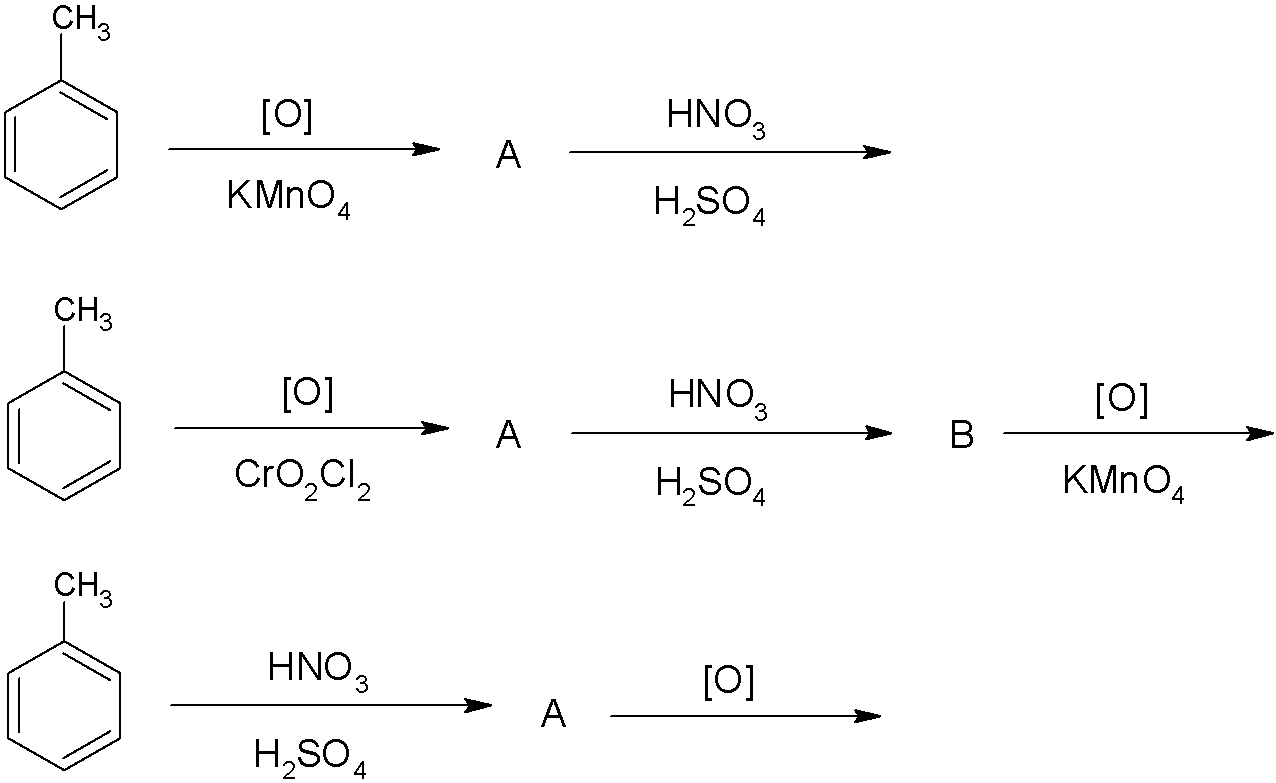
Considering reaction (i) we have the oxidation of toluene by potassium permanganate, which is known to be a strong oxidising agent. As a result, toluene is oxidized to benzoic acid. Benzoic acid is an aromatic compound which is susceptible to electrophilic reaction due to the presence of a highly electronegative and strong electron withdrawing carboxylic group. It withdraws electron resonance and the positive charge density is higher at the ortho and para positions making the meta- position susceptible to electrophilic attack.
Thus, on nitration it will form meta- nitro benzoic acid.
Considering reaction (ii) we have the oxidation of toluene by chromyl chloride, which is known to oxidize toluene to benzaldehyde. Benzaldehyde is an aromatic compound which is susceptible to electrophilic reaction due to the presence of a highly electronegative and strong electron withdrawing carboxyl group. It withdraws electron resonance and the positive charge density is higher at the ortho and para positions making the meta- position susceptible to electrophilic attack.
Thus, on nitration it will form meta- nitro- benzaldehyde which on further oxidation by potassium permanganate will lead to the formation of meta- nitro benzoic acid.
Considering reaction (iii), the first reaction is nitration of toluene. Methyl group is an ortho para directing group and the nitration of toluene would lead to the formation of ortho or para- methyl nitrobenzene, which on further oxidation would give ortho and para benzoic acid respectively.
Thus, the correct answer is A.
Note:
Nitration of aromatic compounds is carried out using a mixture of concentrated nitric acid and concentrated sulphuric acid, known as the nitrating mixture at low temperatures.
Complete answer:
Considering reaction (i) we have the oxidation of toluene by potassium permanganate, which is known to be a strong oxidising agent. As a result, toluene is oxidized to benzoic acid. Benzoic acid is an aromatic compound which is susceptible to electrophilic reaction due to the presence of a highly electronegative and strong electron withdrawing carboxylic group. It withdraws electron resonance and the positive charge density is higher at the ortho and para positions making the meta- position susceptible to electrophilic attack.
Thus, on nitration it will form meta- nitro benzoic acid.
Considering reaction (ii) we have the oxidation of toluene by chromyl chloride, which is known to oxidize toluene to benzaldehyde. Benzaldehyde is an aromatic compound which is susceptible to electrophilic reaction due to the presence of a highly electronegative and strong electron withdrawing carboxyl group. It withdraws electron resonance and the positive charge density is higher at the ortho and para positions making the meta- position susceptible to electrophilic attack.
Thus, on nitration it will form meta- nitro- benzaldehyde which on further oxidation by potassium permanganate will lead to the formation of meta- nitro benzoic acid.
Considering reaction (iii), the first reaction is nitration of toluene. Methyl group is an ortho para directing group and the nitration of toluene would lead to the formation of ortho or para- methyl nitrobenzene, which on further oxidation would give ortho and para benzoic acid respectively.
Thus, the correct answer is A.
Note:
Nitration of aromatic compounds is carried out using a mixture of concentrated nitric acid and concentrated sulphuric acid, known as the nitrating mixture at low temperatures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




