
Lysine is least soluble in water in the pH range:
A.$3$ to $4$
B.$5$ to $6$
C.$6$ to $7$
D.$8$ to $9$
Answer
525k+ views
Hint: We know that there are twenty amino acids in our body which are used in the formation of proteins. We can classify these amino acids as non-essential amino acids, essential amino acids. They are twenty amino acids. Lysine is one among the twenty amino acids and it is an essential amino acid. We can represent Lysine as Lys (or) K.
Complete answer:
We have to know that Lysine comes under an alpha amino acid, which is used in the preparation of plant biosynthesis. It comprises an alpha amino group, an alpha carboxylic acid and lysyl group which acts as a side chain, making it come under basic charge and also aliphatic amino acid. We can draw the skeletal formula of L-Lysine as,
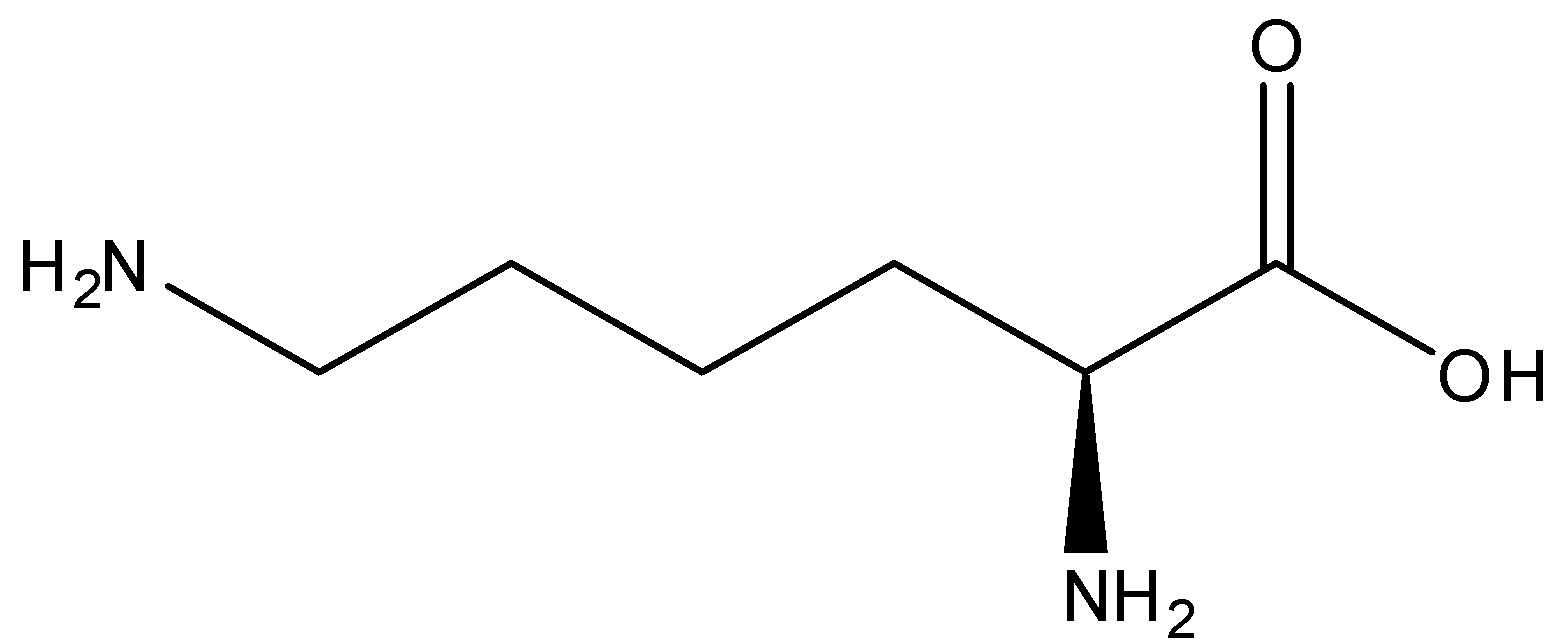
The amino acid Lysine could not be synthesized by the human body. It is important in humans and must be derived from the diet. Catabolism takes place through one of major pathways, and saccharopine pathway is the most common pathway.
We have to know that Lysine is a basic amino acid and so its isoelectric point would lie in the pH range of $8$ to $9$. At this point, the solubility of amino acid lysine in water could be low and we can use this property in the isolation of several amino acids that are derived from protein hydrolysis.
In the pH range of $8$ to $9$, the amino acid lysine is least soluble in water.
Option (D) is correct.
Note:
We have to remember the chemical formula of Lysine as ${C_6}{H_{14}}{N_2}{O_2}$. We have to remember the IUPAC name of L-Lysine as (2S)-2,6-Diaminohexanoic acid) and IUPAC name of D-Lysine is (2R)-2,6-Diaminohexanoic acid). Let us know that the absence of lysine could result in several states of diseases such as defective connective tissues, metabolism of impaired fatty acid, systemic protein-energy deficiency.
Complete answer:
We have to know that Lysine comes under an alpha amino acid, which is used in the preparation of plant biosynthesis. It comprises an alpha amino group, an alpha carboxylic acid and lysyl group which acts as a side chain, making it come under basic charge and also aliphatic amino acid. We can draw the skeletal formula of L-Lysine as,

The amino acid Lysine could not be synthesized by the human body. It is important in humans and must be derived from the diet. Catabolism takes place through one of major pathways, and saccharopine pathway is the most common pathway.
We have to know that Lysine is a basic amino acid and so its isoelectric point would lie in the pH range of $8$ to $9$. At this point, the solubility of amino acid lysine in water could be low and we can use this property in the isolation of several amino acids that are derived from protein hydrolysis.
In the pH range of $8$ to $9$, the amino acid lysine is least soluble in water.
Option (D) is correct.
Note:
We have to remember the chemical formula of Lysine as ${C_6}{H_{14}}{N_2}{O_2}$. We have to remember the IUPAC name of L-Lysine as (2S)-2,6-Diaminohexanoic acid) and IUPAC name of D-Lysine is (2R)-2,6-Diaminohexanoic acid). Let us know that the absence of lysine could result in several states of diseases such as defective connective tissues, metabolism of impaired fatty acid, systemic protein-energy deficiency.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




