
List the following carbocation in order of decreasing stabilization energies:
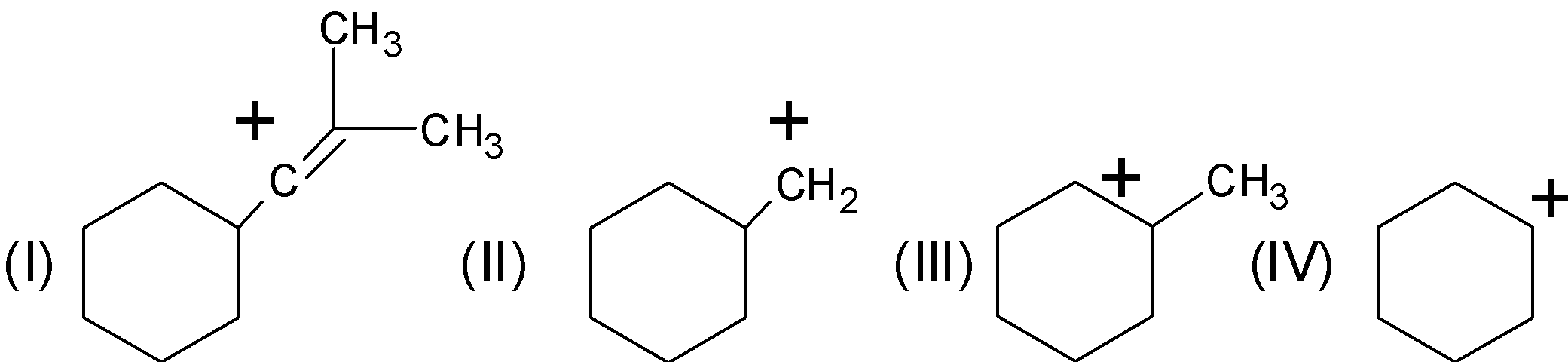
A) $\text{II , III , I , IV}$
B) $\text{III , IV , II , I}$
C) $\text{III , IV , I , III}$
D) $\text{I , II , IV , III}$

Answer
589.2k+ views
Hint: Hyper conjugation is used to depict the stability of the compound. The stability of the carbocation can be measured by the total number of $\text{ }\!\!\alpha\!\!\text{ }$ hydrogen atoms concerning the carbon atom bearing a positive charge. If the structures have the same number of $\text{ }\!\!\alpha\!\!\text{ }$hydrogen atoms then go for the hybridization of the carbocation.
Complete step by step answer:
-The stability of carbocation is related to the energy for a stabilization. More stable carbocation, the less is the stabilization energy.
-Here, we will use the concept of hyperconjugation to determine the stability of carbocation.
-Hyper conjugation is called the stabilization interaction. It arises due to the interaction of electrons in $\sigma $ bond (usually these are $\text{C-H or C-C}$ bonds) with the empty or partially filled p-orbital. This gives the extended molecular structure and increases the stability of the system.
-According to the hyper conjugation, the stability increases with the number of $\alpha -H$ atoms bonded to the carbon forming carbocation. Thus more the number of $\alpha -H$atoms greater is the stability and less is the stabilization energy required.
-Let us have a look at the option.
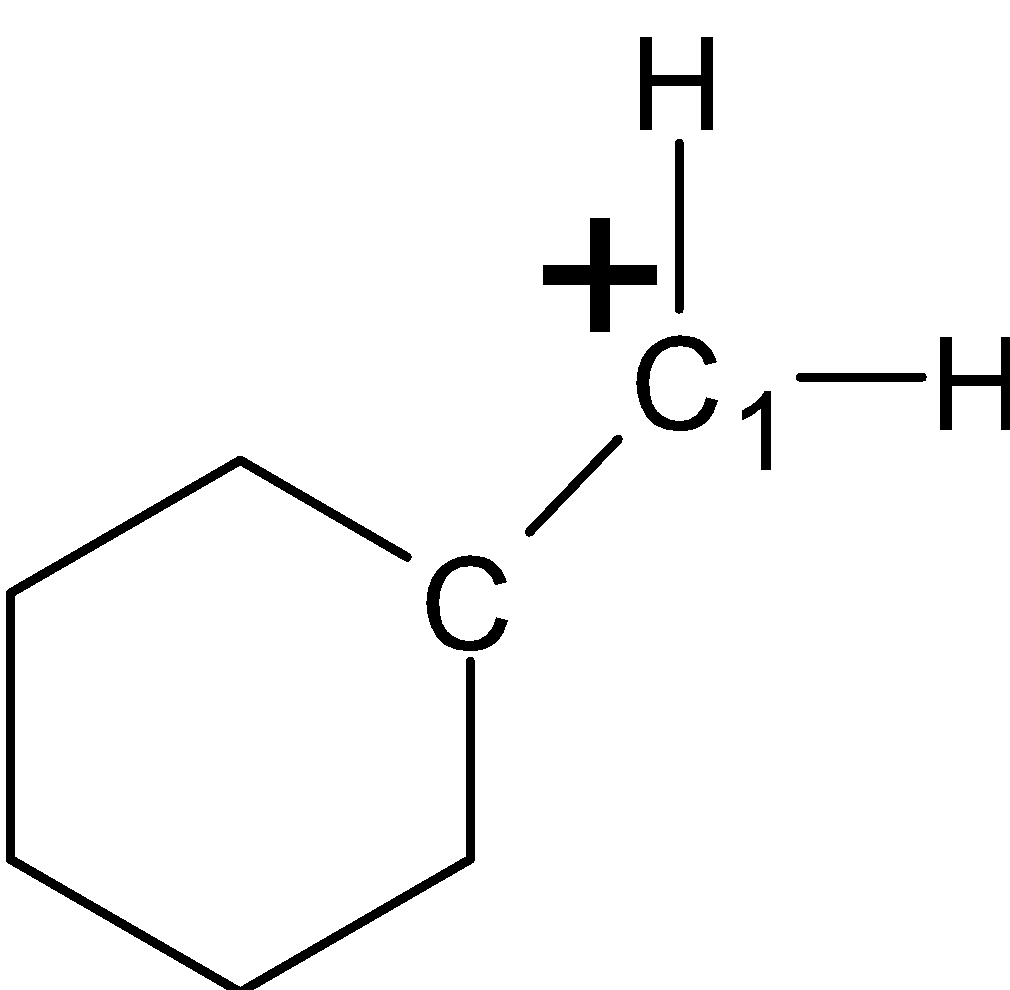
-Here, in the structure (I) the carbocation is formed at the double-bonded carbon${{\text{C}}_{\text{2}}}$. The ${{\text{C}}_{\text{2}}}$carbon is bonded to the one $\alpha -H$atom at the $\text{C}$ring. The $\alpha -H$ as shown below.
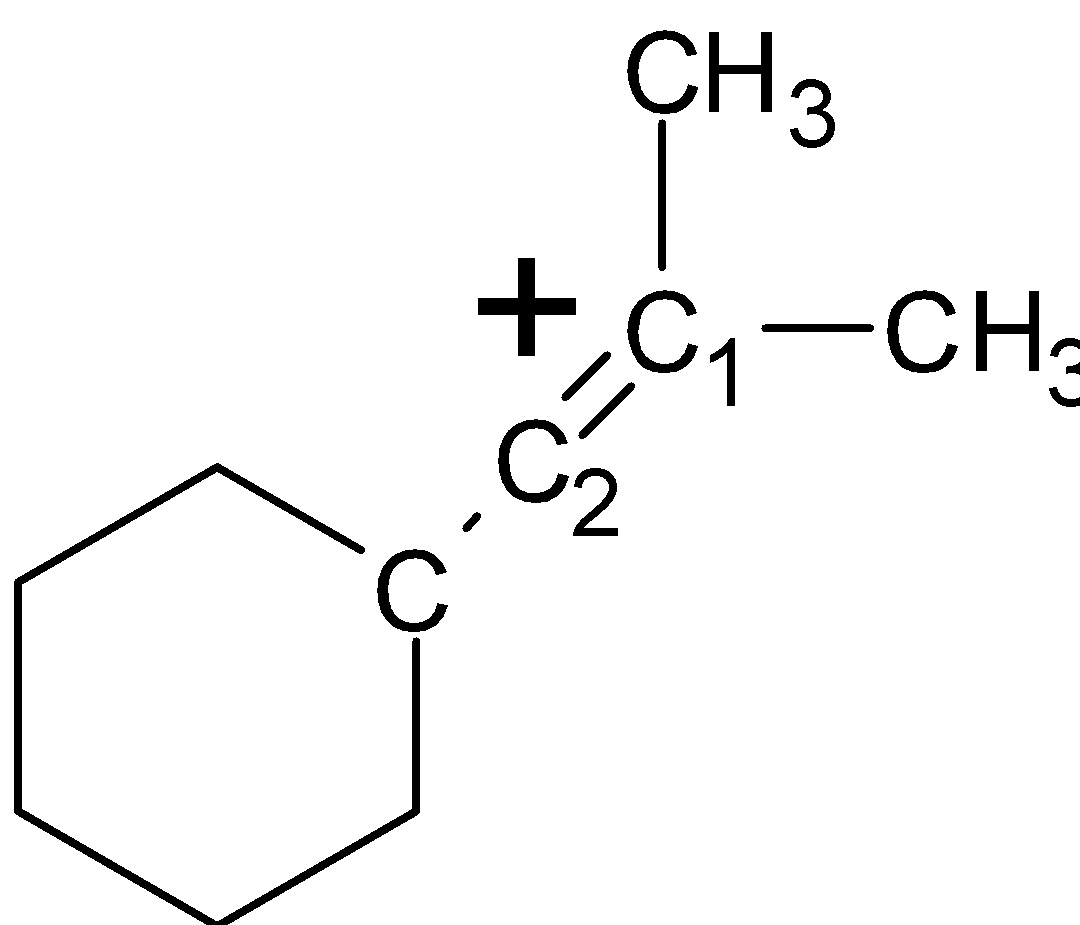
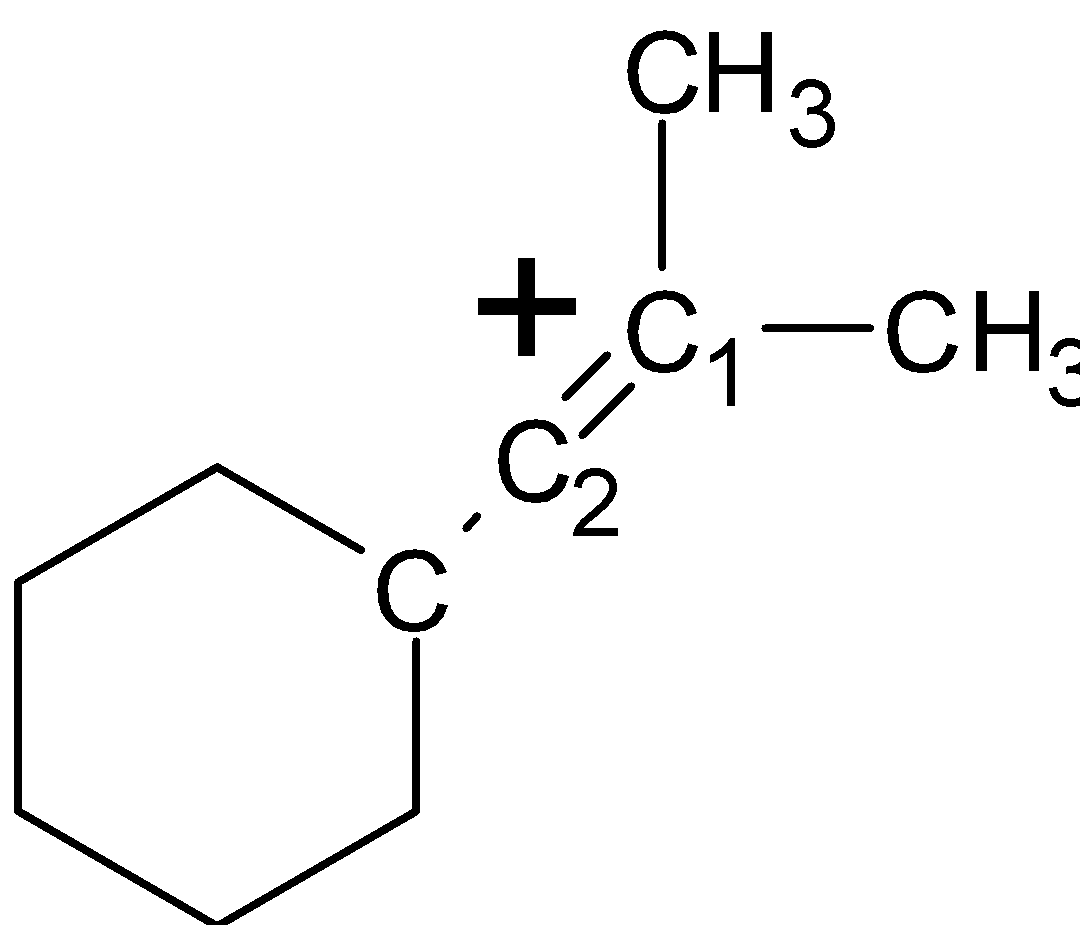
-In structure (III), the carbocation is formed at the ${{\text{C}}_{1}}$ carbon as shown in the structure. There is a total of four $\alpha -H$atoms at the ${{\text{C}}_{\text{2}}}$and ${{\text{C}}_{6}}$ position of the ring. Along with that, there are three more $\alpha -H$atoms at the ${{\text{C}}_{1}}$ carbon atom. Therefore there is a total of 7 $\alpha -H$atoms. As shown below,
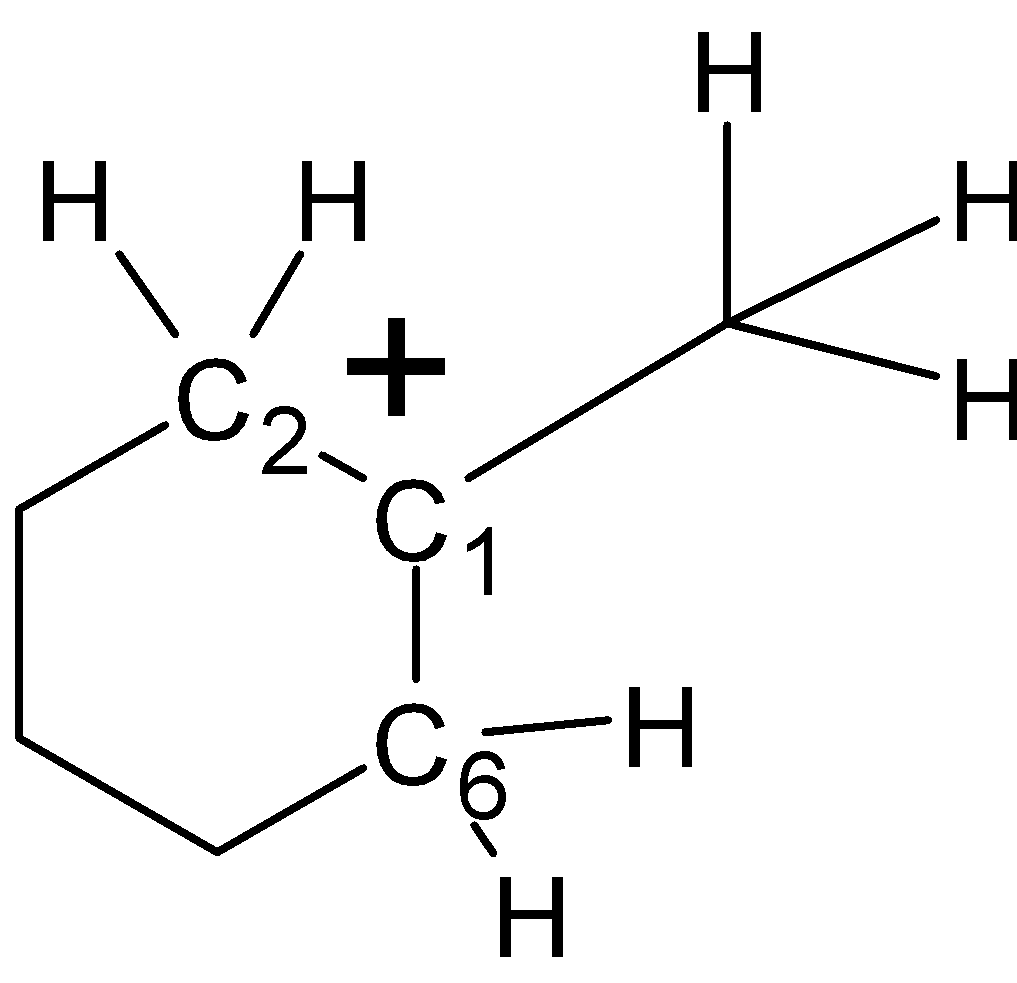
-In structure (IV), let the carbocation is at the ring carbon atom. There are two $\alpha -H$atoms at the ${{\text{C}}_{\text{2}}}$ ring and two at the ${{\text{C}}_{6}}$of the ring .the ${{\text{C}}_{\text{2}}}$and ${{\text{C}}_{6}}$ carbon atoms are at the $\alpha $ to the ${{\text{C}}_{1}}$ carbon. Thus the structure (III) has 4 $\alpha -H$atoms. The structure as shown below,
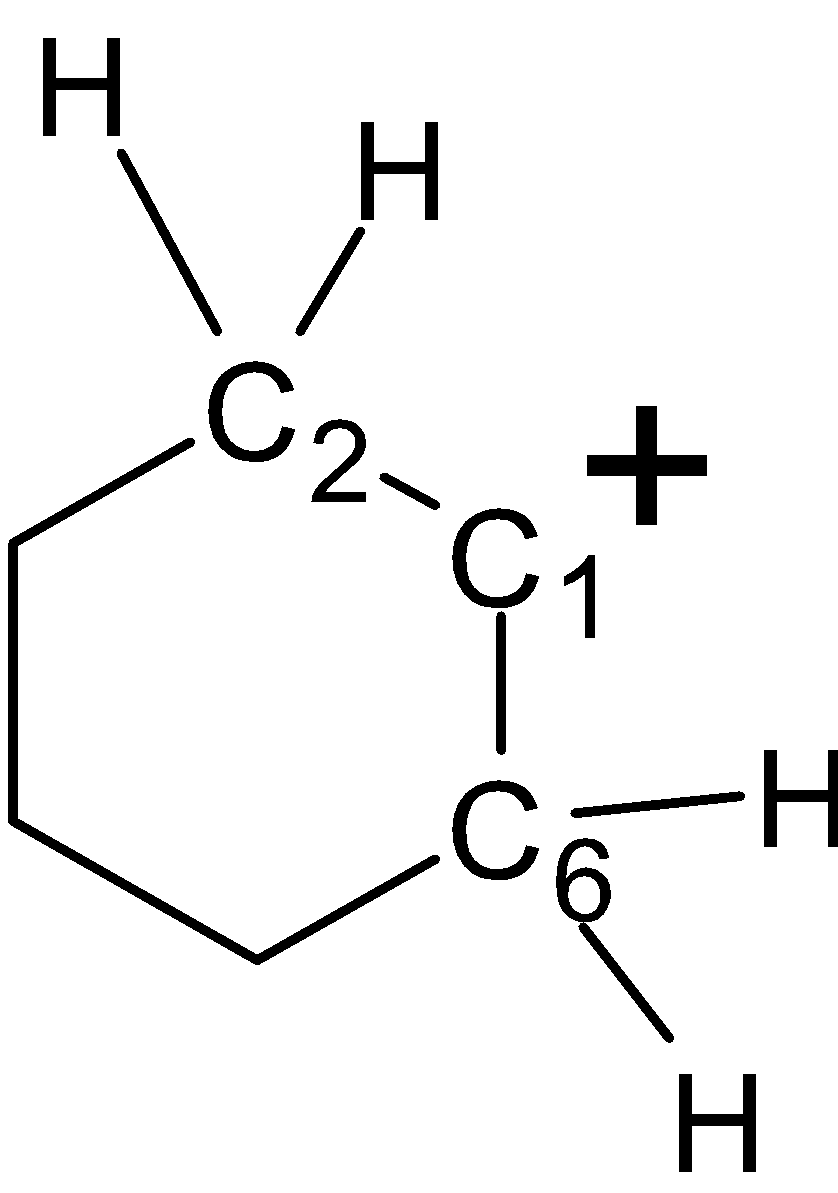
-Thus, the structure (III) is the most stable carbocation followed by the (IV) structure. However, there is still an ambiguity among the structure (I) and (II) as both have the same number of $\alpha -H$ atoms.
Therefore, let us consider the hybridization of the carbon atom. In structure (I), the carbocation is $\text{s}{{\text{p}}^{\text{2}}}$ hybridized which is highly unstable. Therefore (I) will be the least stable of all structures.
Therefore the decreasing order of stability or the stabilization energy is:
$\text{IIIIVIII}$
Hence, (B) is the correct option.
Note: The stability of carbocation also increases as we move from primary to secondary to a tertiary carbon atom.
$\text{Primary (}{{\text{1}}^{\text{0}}}\text{) Secondary (}{{\text{2}}^{\text{0}}}\text{) Tertiary (}{{\text{3}}^{\text{0}}}\text{)}$
Complete step by step answer:
-The stability of carbocation is related to the energy for a stabilization. More stable carbocation, the less is the stabilization energy.
-Here, we will use the concept of hyperconjugation to determine the stability of carbocation.
-Hyper conjugation is called the stabilization interaction. It arises due to the interaction of electrons in $\sigma $ bond (usually these are $\text{C-H or C-C}$ bonds) with the empty or partially filled p-orbital. This gives the extended molecular structure and increases the stability of the system.
-According to the hyper conjugation, the stability increases with the number of $\alpha -H$ atoms bonded to the carbon forming carbocation. Thus more the number of $\alpha -H$atoms greater is the stability and less is the stabilization energy required.
-Let us have a look at the option.
-Here, in the structure (I) the carbocation is formed at the double-bonded carbon${{\text{C}}_{\text{2}}}$. The ${{\text{C}}_{\text{2}}}$carbon is bonded to the one $\alpha -H$atom at the $\text{C}$ring. The $\alpha -H$ as shown below.



-In structure (III), the carbocation is formed at the ${{\text{C}}_{1}}$ carbon as shown in the structure. There is a total of four $\alpha -H$atoms at the ${{\text{C}}_{\text{2}}}$and ${{\text{C}}_{6}}$ position of the ring. Along with that, there are three more $\alpha -H$atoms at the ${{\text{C}}_{1}}$ carbon atom. Therefore there is a total of 7 $\alpha -H$atoms. As shown below,

-In structure (IV), let the carbocation is at the ring carbon atom. There are two $\alpha -H$atoms at the ${{\text{C}}_{\text{2}}}$ ring and two at the ${{\text{C}}_{6}}$of the ring .the ${{\text{C}}_{\text{2}}}$and ${{\text{C}}_{6}}$ carbon atoms are at the $\alpha $ to the ${{\text{C}}_{1}}$ carbon. Thus the structure (III) has 4 $\alpha -H$atoms. The structure as shown below,

-Thus, the structure (III) is the most stable carbocation followed by the (IV) structure. However, there is still an ambiguity among the structure (I) and (II) as both have the same number of $\alpha -H$ atoms.
Therefore, let us consider the hybridization of the carbon atom. In structure (I), the carbocation is $\text{s}{{\text{p}}^{\text{2}}}$ hybridized which is highly unstable. Therefore (I) will be the least stable of all structures.
Therefore the decreasing order of stability or the stabilization energy is:
$\text{IIIIVIII}$
Hence, (B) is the correct option.
Note: The stability of carbocation also increases as we move from primary to secondary to a tertiary carbon atom.
$\text{Primary (}{{\text{1}}^{\text{0}}}\text{) Secondary (}{{\text{2}}^{\text{0}}}\text{) Tertiary (}{{\text{3}}^{\text{0}}}\text{)}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




